Analysis and pharmacological modulation of senescence in human epithelial stem cells
- PMID: 35706382
- PMCID: PMC9279594
- DOI: 10.1111/jcmm.17434
Analysis and pharmacological modulation of senescence in human epithelial stem cells
Abstract
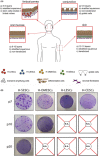
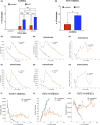
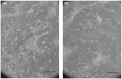
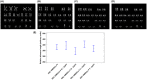
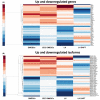
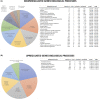
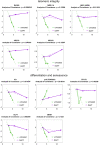
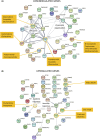
Human epithelial stem cells (ESCs) are characterized by long-term regenerative properties, much dependent on the tissue of origin and varying during their lifespan. We analysed such variables in cultures of ESCs isolated from the skin, conjunctiva, limbus and oral mucosa of healthy donors and patients affected by ectrodactyly-ectodermal dysplasia-clefting syndrome, a rare genetic disorder caused by mutations in the p63 gene. We cultured cells until exhaustion in the presence or in the absence of DAPT (γ-secretase inhibitor; N-[N-(3, 5-difluorophenacetyl)-L-alanyl]-S-phenylglycine T-butyl ester). All cells were able to differentiate in vitro but exhibited variable self-renewal potential. In particular, cells carrying p63 mutations stopped prematurely, compared with controls. Importantly, administration of DAPT significantly extended the replicative properties of all stem cells under examination. RNA sequencing analysis revealed that distinct sets of genes were up- or down-regulated during their lifetime, thus allowing to identify druggable gene networks and off-the-shelf compounds potentially dealing with epithelial stem cell senescence. These data will expand our knowledge on the genetic bases of senescence and potentially pave the way to the pharmacological modulation of ageing in epithelial stem cells.
Keywords: DAPT (γ-secretase inhibitor; N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine T-butyl ester); EEC syndrome; RNAseq; lifespan; p63; self-renewal; stem cells.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures

References
-
- Das D, Fletcher RB, Ngai J. Cellular mechanisms of epithelial stem cell self‐renewal and differentiation during homeostasis and repair. Wiley Interdisciplinary Rev Dev Biol. 2020;9:e361. - PubMed
-
- Rowat JS, Squier CA. Rates of epithelial cell proliferation in the oral mucosa and skin of the tamarin monkey (Saguinus fuscicollis). J Dent Res. 1986;65:1326‐1331. - PubMed
-
- Gibbs S, Ponec M. Intrinsic regulation of differentiation markers in human epidermis, hard palate and buccal mucosa. Arch Oral Biol. 2000;45:149‐158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

