Tips, Tricks, and Potential Pitfalls of CRISPR Genome Editing in Saccharomyces cerevisiae
- PMID: 35706506
- PMCID: PMC9190257
- DOI: 10.3389/fbioe.2022.924914
Tips, Tricks, and Potential Pitfalls of CRISPR Genome Editing in Saccharomyces cerevisiae
Abstract
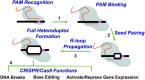
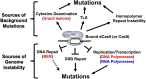
The versatility of clustered regularly interspaced short palindromic repeat (CRISPR)-associated (Cas) genome editing makes it a popular tool for many research and biotechnology applications. Recent advancements in genome editing in eukaryotic organisms, like fungi, allow for precise manipulation of genetic information and fine-tuned control of gene expression. Here, we provide an overview of CRISPR genome editing technologies in yeast, with a particular focus on Saccharomyces cerevisiae. We describe the tools and methods that have been previously developed for genome editing in Saccharomyces cerevisiae and discuss tips and experimental tricks for promoting efficient, marker-free genome editing in this model organism. These include sgRNA design and expression, multiplexing genome editing, optimizing Cas9 expression, allele-specific editing in diploid cells, and understanding the impact of chromatin on genome editing. Finally, we summarize recent studies describing the potential pitfalls of using CRISPR genome targeting in yeast, including the induction of background mutations.
Keywords: Cas9; background mutagenesis; biotechnology; genome engineering; guide RNA; off-target mutagenesis; synthetic biology; yeast.
Copyright © 2022 Antony, Hinz and Wyrick.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

