Dual inhibition of autophagy and PI3K/mTOR pathway as a potential therapeutic strategy against laryngeal squamous cell carcinoma
- PMID: 35706786
- PMCID: PMC9189222
- DOI: 10.21037/tcr-21-2325
Dual inhibition of autophagy and PI3K/mTOR pathway as a potential therapeutic strategy against laryngeal squamous cell carcinoma
Abstract
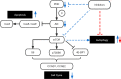
Background: New and effective chemotherapy or targeted therapy strategies are needed against laryngeal squamous cell carcinoma (LSCC). We aimed to explore the antitumor effect of dual PI3K/mTOR inhibitor combined with autophagy suppression on LSCC and its underlying mechanism.
Methods: Hep-2 and AMC-HN-8 cell lines were treated with the Akt inhibitor LY294002, mTOR inhibitor rapamycin, and dual inhibitor NVP-BEZ235 separately. The biological characteristics of in vitro proliferation, cell cycle, apoptosis, migration, invasion, and autophagy were analyzed, and the expression levels of PI3K/Akt/mTOR pathway-related proteins were also measured. The in vivo effects of NVP-BEZ235 combined with inhibition of autophagy using pharmacological inhibitor was further assessed.
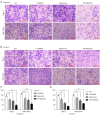
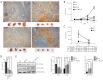
Results: Compared with Akt or mTOR inhibitor, NVP-BEZ235 had the most significant biological effects on LSCC cells. When combined with various autophagy inhibitors, along with siRNA against ATG7, NVP-BEZ235 showed a synergic antitumor effect in LSCC through increasing cell apoptosis and death both in vitro and vivo.
Conclusions: NVP-BEZ235 exerted potent antitumor effects on LSCC, especially when combined with the autophagy inhibitor both in vitro and vivo, providing convincing experimental data for new molecular targeted therapy for LSCC.
Keywords: Laryngeal squamous cell carcinoma (LSCC); PI3K/mTOR inhibitors; autophagy.
2022 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-2325/coif). The authors have no conflicts of interest to declare.
Figures




References
-
- National Comprehensive Cancer Network clinical practice guidelines in oncology, head and neck cancers, version 1.2021. Accessed: 11 March 2021. Available online: https://www.nccn.org/guidelines/patients
LinkOut - more resources
Full Text Sources
Miscellaneous
