APTMS-BCAD modified magnetic iron oxide for magnetic solid-phase extraction of Cu(II) from aqueous solutions
- PMID: 35706942
- PMCID: PMC9189893
- DOI: 10.1016/j.heliyon.2022.e09645
APTMS-BCAD modified magnetic iron oxide for magnetic solid-phase extraction of Cu(II) from aqueous solutions
Abstract
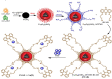
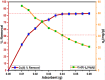
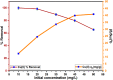
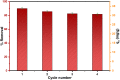
Fe3O4@SiO2-3-aminopropyltrimethoxysilane-1,8-bis (3-chloropropoxy) anthracene-9,10-dione was synthesized as a new, sustainable, and environmentally friendly adsorbent for magnetic solid-phase extraction of Cu(II) from aqueous solutions. The structure of the adsorbent was characterized by FTIR, XRD, SEM, EDX, and TEM analysis. Optimum conditions for Cu(II) adsorption were determined as adsorbent dose 0.04 g, pH 5.0, contact time 120 min, and beginning concentration of 30 mg/L in the adsorption process. The adsorption capacity for Cu(II) ions was 43.67 mg/g and the removal efficiency was 84.72 percent. The Langmuir isotherm and the pseudo-second-order model fit the experimental data better. Adsorption was a spontaneous and endothermic process based on the obtained thermodynamic properties such as ΔG°, ΔH°, and ΔS°. The results showed that the sorbent has good selectivity in the presence of competing ions. The method was determined to be accurate and effective using real water samples and CRM.
Keywords: Adsorption; Cu(II); Isotherm; Kinetic; Magnetic.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Suo L., Dong X., Gao X., Xu J., Huang Z., Ye J., Lu X., Zhao L. Silica-coated magnetic graphene oxide nanocomposite based magnetic solid phase extraction of trace amounts of heavy metals in water samples prior to determination by inductively coupled plasma mass spectrometry. Microc. J. 2019;149
-
- Su S., Chen B., He M., Hu B. Graphene oxide–silica composite coating hollow fiber solid phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta. 2014;123:1–9. - PubMed
-
- Srivastava N., Majumder C. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard Mater. 2008;151:1–8. - PubMed
-
- Dahaghin Z., Mousavi H.Z., Boutorabi L. Application of magnetic ion-imprinted polymer as a new environmentally-friendly nonocomposite for a selective adsorption of the trace level of Cu (II) from aqueous solution and different samples. J. Mol. Liq. 2017;243:380–386.
-
- Tabrizi A.B. Development of a cloud point extraction-spectrofluorimetric method for trace copper (II) determination in water samples and parenteral solutions. J. Hazard Mater. 2007;139(2):260–264. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

