DDQ in mechanochemical C-N coupling reactions
- PMID: 35706992
- PMCID: PMC9174842
- DOI: 10.3762/bjoc.18.64
DDQ in mechanochemical C-N coupling reactions
Abstract
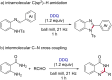
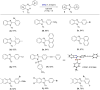
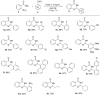
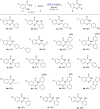
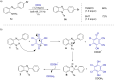
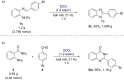
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) is a commonly known oxidant. Herein, we report that DDQ can be used to synthesize 1,2-disubstituted benzimidazoles and quinazolin-4(3H)-ones via the intra- and intermolecular C-N coupling reaction under solvent-free mechanochemical (ball milling) conditions. In the presence of DDQ, the intramolecular C(sp2)-H amidation of N-(2-(arylideneamino)phenyl)-p-toluenesulfonamides leads to 1,2-disubstituted benzimidazoles and the one-pot coupling of 2-aminobenzamides with aryl/alkyl aldehydes resulted in substituted quinazolin-4(3H)-one derivatives in high yields.
Keywords: 1H-benzo[d]imidazole; C(sp2)–H amidation; DDQ; ball mill; mechanochemistry; quinazolin-4(3H)-one.
Copyright © 2022, Bera et al.
Figures
References
LinkOut - more resources
Full Text Sources
