Tamoxifen Increased Parasite Burden and Induced a Series of Histopathological and Immunohistochemical Changes During Chronic Toxoplasmosis in Experimentally Infected Mice
- PMID: 35707167
- PMCID: PMC9189418
- DOI: 10.3389/fmicb.2022.902855
Tamoxifen Increased Parasite Burden and Induced a Series of Histopathological and Immunohistochemical Changes During Chronic Toxoplasmosis in Experimentally Infected Mice
Abstract
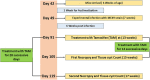
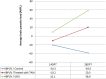
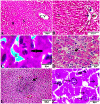
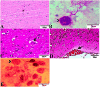
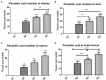
The global distribution of breast cancer and the opportunistic nature of the parasite have resulted in many patients with breast cancer becoming infected with toxoplasmosis. However, very limited information is available about the potential effects of tamoxifen on chronic toxoplasmosis and its contribution to the reactivation of the latent infection. The present study investigated the potential effects of tamoxifen on chronic toxoplasmosis in animal models (Swiss albino mice). Following induction of chronic toxoplasmosis and treatment with the drug for 14 and 28 days, the anti-parasitic effects of tamoxifen were evaluated by parasitological assessment and counting of Toxoplasma cysts. In addition, the effects of the drug on the parasite load were evaluated and quantitated using TaqMan real-time quantitative PCR followed by investigation of the major histopathological changes and immunohistochemical findings. Interestingly, tamoxifen increased the parasite burden on animals treated with the drug during 14 and 28 days as compared with the control group. The quantification of the DNA concentrations of Toxoplasma P29 gene after the treatment with the drug revealed a higher parasite load in both treated groups vs. control groups. Furthermore, treatment with tamoxifen induced a series of histopathological and immunohistochemical changes in the kidney, liver, brain, and uterus, revealing the exacerbating effect of tamoxifen against chronic toxoplasmosis. These changes were represented by the presence of multiple T. gondii tissue cysts in the lumen of proximal convoluted tubules associated with complete necrosis in their lining epithelium of the kidney section. Meanwhile, liver tissue revealed multiple T. gondii tissue cysts in hepatic parenchyma which altered the structure of hepatocytes. Moreover, clusters of intracellular tachyzoites were observed in the lining epithelium of endometrium associated with severe endometrial necrosis and appeared as diffuse nuclear pyknosis combined with sever mononuclear cellular infiltration. Brain tissues experienced the presence of hemorrhages in pia mater and multiple T. gondii tissue cysts in brain tissue. The severity of the lesions was maximized by increasing the duration of treatment. Collectively, the study concluded novel findings in relation to the potential role of tamoxifen during chronic toxoplasmosis. These findings are very important for combating the disease, particularly in immunocompromised patients which could be life-threatening.
Keywords: chronic toxoplasmosis; histopathology; immunohistochemistry; real-time PCR; tamoxifen.
Copyright © 2022 Barakat, El Fadaly, Selem, Madboli, Abd El-Razik, Hassan, Alghamdi and Elmahallawy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bancroft J. D., Gamble M. (2008). Theory and Practice of Histological Techniques. 6th Edn. Churchill Livingstone, London, United Kingdom: Elsevier Health Sciences.
LinkOut - more resources
Full Text Sources

