Molecular and Biochemical Analysis of Duplicated Cytosolic CuZn Superoxide Dismutases of Rice and in silico Analysis in Plants
- PMID: 35707617
- PMCID: PMC9191229
- DOI: 10.3389/fpls.2022.864330
Molecular and Biochemical Analysis of Duplicated Cytosolic CuZn Superoxide Dismutases of Rice and in silico Analysis in Plants
Abstract
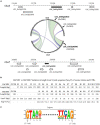
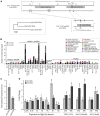
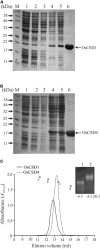
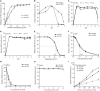
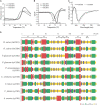
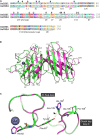
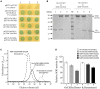
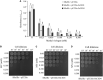
Superoxide dismutases (SODs, EC 1.15.1.1) are ubiquitous antioxidant metalloenzymes important for oxidative stress tolerance and cellular redox environment. Multiple factors have contributed toward the origin and diversity of SOD isoforms among different organisms. In plants, the genome duplication events, responsible for the generation of multiple gene copies/gene families, have also contributed toward the SOD diversity. However, the importance of such molecular events on the characteristics of SODs has not been studied well. This study investigated the effects of divergence on important characteristics of two block-duplicated rice cytosolic CuZn SODs (OsCSD1, OsCSD4), along with in silico assessment of similar events in other plants. The analysis revealed heterogeneity in gene length, regulatory regions, untranslated regions (UTRs), and coding regions of two OsCSDs. An inconsistency in the database-predicted OsCSD1 gene structure was also identified and validated experimentally. Transcript analysis showed differences in the basal levels and stress responsiveness of OsCSD1 and OsCSD4, and indicated the presence of two transcription start sites in the OsCSD1. At the amino acid level, the two OsCSDs showed differences at 18 sites; however, both exist as a homodimer, displaying typical CuZn SOD characteristics, and enhancing the oxidative stress tolerance of Escherichia coli cells. However, OsCSD4 showed higher specific activity as well as stability. The comparison of the two OsCSDs with reported thermostable CSDs from other plants identified regions likely to be associated with stability, while the homology modeling and superposition highlighted structural differences. The two OsCSDs displayed heteromeric interaction capability and forms an enzymatically active heterodimer (OsCSD1:OsCSD4) on co-expression, which may have significance as both are cytosolic. In silico analysis of 74 plant genomes revealed the prevalence of block duplications for multiple CSD copies (mostly cytosolic). The divergence and clustering analysis of CSDs suggested the possibility of an ancestral duplication event in monocots. Conserved SOD features indicating retention of SOD function among CSD duplicates were evident in few monocots and dicots. In most other species, the CSD copies lacked critical features and may not harbor SOD function; however, other feature-associated functions or novel functions might be present. These aspects of divergent CSD copies encoding co-localized CSDs may have implications in plant SOD functions in the cytosol and other organelles.
Keywords: Oryza sativa; block duplication; cytosolic CuZn superoxide dismutase; heteromeric interaction; homology modeling; in silico analysis; oxidative stress; thermostability.
Copyright © 2022 Sanyal, Prashar, Jawali, Sunkar, Misra and Saini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Barker M. S., Baute G. J., Liu S. L. (2012). “Duplications and turnover in plant genomes,” in Plant Genome Diversity, Vol. 1 eds Wendel J., Greilhuber J., Doleze J., Leitch I. (Vienna: Springer; ), 155–169. 10.1007/978-3-7091-1130-7_11 - DOI
LinkOut - more resources
Full Text Sources

