Enabling Allogeneic T Cell-Based Therapies: Scalable Stirred-Tank Bioreactor Mediated Manufacturing
- PMID: 35707712
- PMCID: PMC9189297
- DOI: 10.3389/fmedt.2022.850565
Enabling Allogeneic T Cell-Based Therapies: Scalable Stirred-Tank Bioreactor Mediated Manufacturing
Abstract
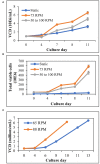
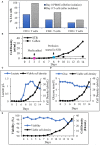
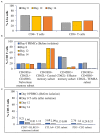
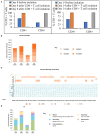
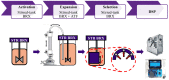
Allogeneic T cells are key immune therapeutic cells to fight cancer and other clinical indications. High T cell dose per patient and increasing patient numbers result in clinical demand for a large number of allogeneic T cells. This necessitates a manufacturing platform that can be scaled up while retaining cell quality. Here we present a closed and scalable platform for T cell manufacturing to meet clinical demand. Upstream manufacturing steps of T cell activation and expansion are done in-vessel, in a stirred-tank bioreactor. T cell selection, which is necessary for CAR-T-based therapy, is done in the bioreactor itself, thus maintaining optimal culture conditions through the selection step. Platform's attributes of automation and performing the steps of T cell activation, expansion, and selection in-vessel, greatly contribute to enhancing process control, cell quality, and to the reduction of manual labor and contamination risk. In addition, the viability of integrating a closed, automated, downstream process of cell concentration, is demonstrated. The presented T cell manufacturing platform has scale-up capabilities while preserving key factors of cell quality and process control.
Keywords: T cell manufacturing; allogeneic T cells; automation; bioreactor; closed process.
Copyright © 2022 Gatla, Uth, Levinson, Navaei, Sargent, Ramaswamy and Friedrich Ben-Nun.
Conflict of interest statement
All authors are current or previous employees of Lonza, a pharmaceutical company that develops and sells a wide range of products, including cell-based products for research and pharmaceutical use. Lonza may derive benefit from the sale of a product derived from this research. However, this does not alter our adherence to all the IJMS policies on sharing data and materials.
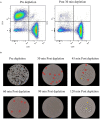
Figures
References
LinkOut - more resources
Full Text Sources

