A cohort study of gill infections, gill pathology and gill-related mortality in sea-farmed Atlantic salmon (Salmo salar L.): A descriptive analysis
- PMID: 35707921
- PMCID: PMC9543555
- DOI: 10.1111/jfd.13662
A cohort study of gill infections, gill pathology and gill-related mortality in sea-farmed Atlantic salmon (Salmo salar L.): A descriptive analysis
Abstract
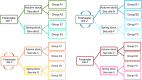
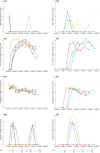
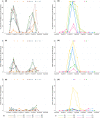
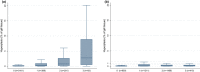
Gill disease is an important cause of economic losses, fish mortality and reduced animal welfare in salmonid farming. We performed a prospective cohort study, following groups of Atlantic salmon in Western Norway with repeated sampling and data collection from the hatchery phase and throughout the 1st year at sea. The objective was to determine if variation in pathogen prevalence and load, and zoo- and phytoplankton levels had an impact on gill health. Further to describe the temporal development of pathogen prevalence and load, and gill pathology, and how these relate to each other. Neoparamoeba perurans appeared to be the most important cause of gill pathology. No consistent covariation and no or weak associations between the extent of gill pathology and prevalence and load of SGPV, Ca. B. cysticola and D. lepeophtherii were observed. At sea, D. lepeophtherii and Ca. B. cysticola persistently infected all fish groups. Fish groups negative for SGPV at sea transfer were infected at sea and fish groups tested negative before again testing positive. This is suggestive of horizontal transmission of infection at sea and may indicate that previous SGPV infection does not protect against reinfection. Coinfections with three or more putative gill pathogens were found in all fish groups and appear to be the norm in sea-farmed Atlantic salmon in Western Norway.
Keywords: D. lepeophtherii; AGD; Atlantic salmon; Ca. B. cysticola; SGPV; gill disease.
© 2022 The Authors. Journal of Fish Diseases published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Prospective Longitudinal Study of Putative Agents Involved in Complex Gill Disorder in Atlantic salmon (Salmo salar).Pathogens. 2022 Aug 3;11(8):878. doi: 10.3390/pathogens11080878. Pathogens. 2022. PMID: 36014998 Free PMC article.
-
Complex Gill Disease: an Emerging Syndrome in Farmed Atlantic Salmon (Salmo salar L.).J Comp Pathol. 2018 Aug;163:23-28. doi: 10.1016/j.jcpa.2018.07.004. Epub 2018 Aug 16. J Comp Pathol. 2018. PMID: 30213370 Review.
-
Histopathological investigation of complex gill disease in sea farmed Atlantic salmon.PLoS One. 2019 Oct 3;14(10):e0222926. doi: 10.1371/journal.pone.0222926. eCollection 2019. PLoS One. 2019. PMID: 31581255 Free PMC article.
-
Amoebic gill disease and host response in Atlantic salmon (Salmo salar L.): A review.Parasite Immunol. 2020 Aug;42(8):e12766. doi: 10.1111/pim.12766. Epub 2020 Jul 13. Parasite Immunol. 2020. PMID: 32564378 Review.
-
Mucosal and Systemic Immune Responses to Salmon Gill Poxvirus Infection in Atlantic Salmon Are Modulated Upon Hydrocortisone Injection.Front Immunol. 2021 Jun 9;12:689302. doi: 10.3389/fimmu.2021.689302. eCollection 2021. Front Immunol. 2021. PMID: 34177946 Free PMC article.
Cited by
-
Assessment of a semiquantitative scoring system for mild-to-moderate gill lesions in Atlantic salmon reared in recirculating aquaculture systems in Norway.J Vet Diagn Invest. 2025 Mar;37(2):252-262. doi: 10.1177/10406387241310900. Epub 2025 Jan 28. J Vet Diagn Invest. 2025. PMID: 39876026 Free PMC article.
-
Emergence of Salmon Gill Poxvirus.Viruses. 2022 Dec 1;14(12):2701. doi: 10.3390/v14122701. Viruses. 2022. PMID: 36560705 Free PMC article. Review.
-
Effectiveness of functional ingredients to enhance gill disease in Atlantic salmon (Salmo salar, L.).PLoS One. 2024 Jun 20;19(6):e0304112. doi: 10.1371/journal.pone.0304112. eCollection 2024. PLoS One. 2024. PMID: 38900829 Free PMC article.
-
Diseases of marine fish and shellfish in an age of rapid climate change.iScience. 2024 Aug 28;27(9):110838. doi: 10.1016/j.isci.2024.110838. eCollection 2024 Sep 20. iScience. 2024. PMID: 39318536 Free PMC article. Review.
References
-
- Amundsen, M. M. , Tartor, H. , Andersen, K. , Sveinsson, K. , Thoen, E. , Gjessing, M. C. , & Dahle, M. K. (2021). Mucosal and systemic immune responses to salmon gill poxvirus infection in Atlantic salmon is modulated upon hydrocortisone injection. Frontiers in Immunology, 12. 10.3389/fimmu.2021.689302 - DOI - PMC - PubMed
-
- Båmstedt, U. , Fosså, J. H. , Martinussen, M. B. , & Fosshagen, A. (2012). Mass occurrence of the physonect siphonophore Apolemia uvaria (Lesueur) in Norwegian waters. Sarsia, 83(1), 79–85. 10.1080/00364827.1998.10413673 - DOI
-
- Baxter, E. J. , Rodger, H. D. , McAllen, R. , & Doyle, T. K. (2011). Gill disorders in marine‐farmed salmon: investigating the role of hydrozoan jellyfish. Aquaculture Environment Interactions, 1(3), 245–257. 10.3354/aei00024 - DOI
-
- Baxter, E. J. , Sturt, M. M. , Ruane, N. M. , Doyle, T. K. , McAllen, R. , Harman, L. , & Rodger, H. D. (2011). Gill damage to Atlantic salmon (Salmo salar) caused by the common jellyfish (Aurelia aurita) under experimental challenge. PLoS One, 6(4), e18529. 10.1371/journal.pone.0018529 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

