Effect of Fluid Bolus Administration on Cardiovascular Collapse Among Critically Ill Patients Undergoing Tracheal Intubation: A Randomized Clinical Trial
- PMID: 35707974
- PMCID: PMC9204618
- DOI: 10.1001/jama.2022.9792
Effect of Fluid Bolus Administration on Cardiovascular Collapse Among Critically Ill Patients Undergoing Tracheal Intubation: A Randomized Clinical Trial
Abstract
Importance: Hypotension is common during tracheal intubation of critically ill adults and increases the risk of cardiac arrest and death. Whether administering an intravenous fluid bolus to critically ill adults undergoing tracheal intubation prevents severe hypotension, cardiac arrest, or death remains uncertain.
Objective: To determine the effect of fluid bolus administration on the incidence of severe hypotension, cardiac arrest, and death.
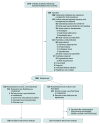
Design, setting, and participants: This randomized clinical trial enrolled 1067 critically ill adults undergoing tracheal intubation with sedation and positive pressure ventilation at 11 intensive care units in the US between February 1, 2019, and May 24, 2021. The date of final follow-up was June 21, 2021.
Interventions: Patients were randomly assigned to receive either a 500-mL intravenous fluid bolus (n = 538) or no fluid bolus (n = 527).
Main outcomes and measures: The primary outcome was cardiovascular collapse (defined as new or increased receipt of vasopressors or a systolic blood pressure <65 mm Hg between induction of anesthesia and 2 minutes after tracheal intubation, or cardiac arrest or death between induction of anesthesia and 1 hour after tracheal intubation). The secondary outcome was the incidence of death prior to day 28, which was censored at hospital discharge.
Results: Among 1067 patients randomized, 1065 (99.8%) completed the trial and were included in the primary analysis (median age, 62 years [IQR, 51-70 years]; 42.1% were women). Cardiovascular collapse occurred in 113 patients (21.0%) in the fluid bolus group and in 96 patients (18.2%) in the no fluid bolus group (absolute difference, 2.8% [95% CI, -2.2% to 7.7%]; P = .25). New or increased receipt of vasopressors occurred in 20.6% of patients in the fluid bolus group compared with 17.6% of patients in the no fluid bolus group, a systolic blood pressure of less than 65 mm Hg occurred in 3.9% vs 4.2%, respectively, cardiac arrest occurred in 1.7% vs 1.5%, and death occurred in 0.7% vs 0.6%. Death prior to day 28 (censored at hospital discharge) occurred in 218 patients (40.5%) in the fluid bolus group compared with 223 patients (42.3%) in the no fluid bolus group (absolute difference, -1.8% [95% CI, -7.9% to 4.3%]; P = .55).
Conclusions and relevance: Among critically ill adults undergoing tracheal intubation, administration of an intravenous fluid bolus compared with no fluid bolus did not significantly decrease the incidence of cardiovascular collapse.
Trial registration: ClinicalTrials.gov Identifier: NCT03787732.
Conflict of interest statement
Figures

Comment in
-
A fluid bolus before tracheal intubation in the critically ill does not prevent peri-intubation cardiovascular collapse: Time to consider alternatives?Anaesth Crit Care Pain Med. 2022 Dec;41(6):101158. doi: 10.1016/j.accpm.2022.101158. Epub 2022 Sep 12. Anaesth Crit Care Pain Med. 2022. PMID: 36100061 No abstract available.
-
Ill PREPAREd-II Intubate the Critically Ill: October 2022 Annals of Emergency Medicine Journal Club.Ann Emerg Med. 2022 Oct;80(4):377-378. doi: 10.1016/j.annemergmed.2022.08.013. Ann Emerg Med. 2022. PMID: 36153051 No abstract available.
-
Fluid Bolus Administration and Cardiovascular Collapse in Critically Ill Patients Undergoing Tracheal Intubation.JAMA. 2022 Nov 22;328(20):2070. doi: 10.1001/jama.2022.17505. JAMA. 2022. PMID: 36413238 No abstract available.
-
Fluid Bolus Administration and Cardiovascular Collapse in Critically Ill Patients Undergoing Tracheal Intubation.JAMA. 2022 Nov 22;328(20):2069-2070. doi: 10.1001/jama.2022.17502. JAMA. 2022. PMID: 36413239 No abstract available.
-
Fluid Bolus Administration and Cardiovascular Collapse in Critically Ill Patients Undergoing Tracheal Intubation.JAMA. 2022 Nov 22;328(20):2070-2071. doi: 10.1001/jama.2022.17499. JAMA. 2022. PMID: 36413240 No abstract available.
References
-
- Janz DR, Casey JD, Semler MW, et al. ; PrePARE Investigators; Pragmatic Critical Care Research Group . Effect of a fluid bolus on cardiovascular collapse among critically ill adults undergoing tracheal intubation (PrePARE): a randomised controlled trial. Lancet Respir Med. 2019;7(12):1039-1047. doi: 10.1016/S2213-2600(19)30246-2 - DOI - PMC - PubMed

