Effect of High-Flow Nasal Cannula Therapy vs Continuous Positive Airway Pressure Therapy on Liberation From Respiratory Support in Acutely Ill Children Admitted to Pediatric Critical Care Units: A Randomized Clinical Trial
- PMID: 35707984
- PMCID: PMC9204623
- DOI: 10.1001/jama.2022.9615
Effect of High-Flow Nasal Cannula Therapy vs Continuous Positive Airway Pressure Therapy on Liberation From Respiratory Support in Acutely Ill Children Admitted to Pediatric Critical Care Units: A Randomized Clinical Trial
Abstract
Importance: The optimal first-line mode of noninvasive respiratory support for acutely ill children is not known.
Objective: To evaluate the noninferiority of high-flow nasal cannula therapy (HFNC) as the first-line mode of noninvasive respiratory support for acute illness, compared with continuous positive airway pressure (CPAP), for time to liberation from all forms of respiratory support.
Design, setting, and participants: Pragmatic, multicenter, randomized noninferiority clinical trial conducted in 24 pediatric critical care units in the United Kingdom among 600 acutely ill children aged 0 to 15 years who were clinically assessed to require noninvasive respiratory support, recruited between August 2019 and November 2021, with last follow-up completed in March 2022.
Interventions: Patients were randomized 1:1 to commence either HFNC at a flow rate based on patient weight (n = 301) or CPAP of 7 to 8 cm H2O (n = 299).
Main outcomes and measures: The primary outcome was time from randomization to liberation from respiratory support, defined as the start of a 48-hour period during which a participant was free from all forms of respiratory support (invasive or noninvasive), assessed against a noninferiority margin of an adjusted hazard ratio of 0.75. Seven secondary outcomes were assessed, including mortality at critical care unit discharge, intubation within 48 hours, and use of sedation.
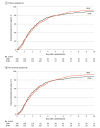
Results: Of the 600 randomized children, consent was not obtained for 5 (HFNC: 1; CPAP: 4) and respiratory support was not started in 22 (HFNC: 5; CPAP: 17); 573 children (HFNC: 295; CPAP: 278) were included in the primary analysis (median age, 9 months; 226 girls [39%]). The median time to liberation in the HFNC group was 52.9 hours (95% CI, 46.0-60.9 hours) vs 47.9 hours (95% CI, 40.5-55.7 hours) in the CPAP group (absolute difference, 5.0 hours [95% CI -10.1 to 17.4 hours]; adjusted hazard ratio 1.03 [1-sided 97.5% CI, 0.86-∞]). This met the criterion for noninferiority. Of the 7 prespecified secondary outcomes, 3 were significantly lower in the HFNC group: use of sedation (27.7% vs 37%; adjusted odds ratio, 0.59 [95% CI, 0.39-0.88]); mean duration of critical care stay (5 days vs 7.4 days; adjusted mean difference, -3 days [95% CI, -5.1 to -1 days]); and mean duration of acute hospital stay (13.8 days vs 19.5 days; adjusted mean difference, -7.6 days [95% CI, -13.2 to -1.9 days]). The most common adverse event was nasal trauma (HFNC: 6/295 [2.0%]; CPAP: 18/278 [6.5%]).
Conclusions and relevance: Among acutely ill children clinically assessed to require noninvasive respiratory support in a pediatric critical care unit, HFNC compared with CPAP met the criterion for noninferiority for time to liberation from respiratory support.
Trial registration: ISRCTN.org Identifier: ISRCTN60048867.
Conflict of interest statement
Figures

References
-
- Paediatric Intensive Care Audit Network (PICANet) . Annual Report 2020. PICANet; 2020.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

