Molecular mechanisms of cystic fibrosis - how mutations lead to misfunction and guide therapy
- PMID: 35707985
- PMCID: PMC9251585
- DOI: 10.1042/BSR20212006
Molecular mechanisms of cystic fibrosis - how mutations lead to misfunction and guide therapy
Abstract
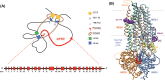
Cystic fibrosis, the most common autosomal recessive disorder in Caucasians, is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes a cAMP-activated chloride and bicarbonate channel that regulates ion and water transport in secretory epithelia. Although all mutations lead to the lack or reduction in channel function, the mechanisms through which this occurs are diverse - ranging from lack of full-length mRNA, reduced mRNA levels, impaired folding and trafficking, targeting to degradation, decreased gating or conductance, and reduced protein levels to decreased half-life at the plasma membrane. Here, we review the different molecular mechanisms that cause cystic fibrosis and detail how these differences identify theratypes that can inform the use of directed therapies aiming at correcting the basic defect. In summary, we travel through CFTR life cycle from the gene to function, identifying what can go wrong and what can be targeted in terms of the different types of therapeutic approaches.
Keywords: CFTR; Cystic Fibrosis; Molecular basis of disease; Mutations; Theratypes.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

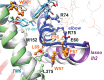

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

