Centriole distal-end proteins CP110 and Cep97 influence centriole cartwheel growth at the proximal end
- PMID: 35707992
- PMCID: PMC9450887
- DOI: 10.1242/jcs.260015
Centriole distal-end proteins CP110 and Cep97 influence centriole cartwheel growth at the proximal end
Abstract
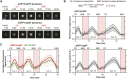
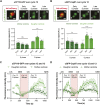
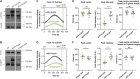
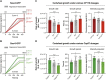
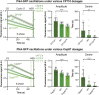
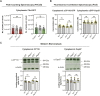
Centrioles are composed of a central cartwheel tethered to nine-fold symmetric microtubule (MT) blades. The centriole cartwheel and MTs are thought to grow from opposite ends of these organelles, so it is unclear how they coordinate their assembly. We previously showed that in Drosophila embryos an oscillation of Polo-like kinase 4 (Plk4) helps to initiate and time the growth of the cartwheel at the proximal end. Here, in the same model, we show that CP110 and Cep97 form a complex close to the distal-end of the centriole MTs whose levels rise and fall as the new centriole MTs grow, in a manner that appears to be entrained by the core cyclin-dependent kinase (Cdk)-Cyclin oscillator that drives the nuclear divisions in these embryos. These CP110 and Cep97 dynamics, however, do not appear to time the period of centriole MT growth directly. Instead, we find that changing the levels of CP110 and Cep97 appears to alter the Plk4 oscillation and the growth of the cartwheel at the proximal end. These findings reveal an unexpected potential crosstalk between factors normally concentrated at opposite ends of the growing centrioles, which might help to coordinate centriole growth. This article has an associated First Person interview with the first authors of the paper.
Keywords: CP110; Cell cycle; Centriole; Centrosome; Cep97; Embryogenesis; Organelle biogenesis; Polo-like kinase 4.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests Laura Hankins is currently an employee of The Company of Biologists and had no role in the review of the paper. The authors declare no other competing interests for this study.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

