Follow-up evaluation of patients with liver test abnormalities detected during SARS-CoV2 infection
- PMID: 35708160
- PMCID: PMC9350227
- DOI: 10.1111/jvh.13718
Follow-up evaluation of patients with liver test abnormalities detected during SARS-CoV2 infection
Abstract
Abnormal liver function tests (A-LFTs) during admission for coronavirus disease-19 (COVID-19) are frequent, but its evolution after COVID-19 resolution remains unexplored. We evaluated factors related to A-LFTs during COVID-19 and assessed the liver outcome after patients' discharge. This is a observational study including: (1) retrospective analysis of variables related to A-LFTs during COVID-19; and (2) follow-up evaluation with blood test, transient elastography and liver biopsy in those with persistent A-LFTs. A-LFTs were defined according to CTCAEv4.0. Among 595 patients, 366 (61.5%) showed A-LFTs. The ratio of partial pressure of oxygen and inspired oxygen fraction (P/F) below 200, ferritin ≥1000 ng/mL, male gender and antibiotic and immunomodulatory treatments were related to A-LFTs. Follow-up evaluation was performed in 153 individuals. Persistent A-LFTs at follow-up was similar in patients with/without A-LFTs during admission (14.1% vs. 4.9%, p = 0.104). Fifteen (93%) and 58 (39%) patients with/without A-LFTs at follow-up showed metabolic fatty liver disease criteria (p < 0.001), which were histologically confirmed. In conclusion, A-LFTs during COVID-19 were related to infection severity. Abnormalities remitted at follow-up in >80% of patients, and no correlation between A-LFTs at admission and at follow-up was found. Most patients with A-LFTs at follow-up had non-invasive and histologically proven fatty liver disease.
Keywords: COVID-19; SARS-CoV2; follow-up; liver function tests; metabolic-associated fatty liver disease (MAFLD).
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest relevant to this work.
Figures
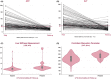

Comment in
-
Reply to: 'Abnormal liver function tests and coronavirus disease 2019: A close relationship'.J Viral Hepat. 2023 Jan;30(1):81. doi: 10.1111/jvh.13773. Epub 2022 Nov 21. J Viral Hepat. 2023. PMID: 36367344 Free PMC article. No abstract available.
-
Abnormal liver function tests and coronavirus disease 2019: A close relationship.J Viral Hepat. 2023 Jan;30(1):79-80. doi: 10.1111/jvh.13772. Epub 2022 Nov 21. J Viral Hepat. 2023. PMID: 36369662 Free PMC article. No abstract available.
References
-
- Ritchie H, Ortiz‐Ospina E, Beltekian D, et al. Our World In Data. OurWorldInData.org. https://ourworldindata.org/coronavirus. Accessed June 1, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

