Cerebellar contribution to threat probability in a SCA6 mouse model
- PMID: 35708512
- PMCID: PMC9652111
- DOI: 10.1093/hmg/ddac135
Cerebellar contribution to threat probability in a SCA6 mouse model
Abstract
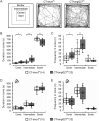
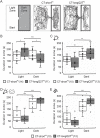
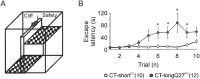
Fear and anxiety have proven to be essential during the evolutionary process. However, the mechanisms involved in recognizing and categorizing threat probability (i.e. low to high) to elicit the appropriate defensive behavior are yet to be determined. In this study, we investigated the cerebellar contribution in evoking appropriate defensive escape behavior using a purely cerebellar, neurodegenerative mouse model for spinocerebellar ataxia type 6 which is caused by an expanded CAG repeat in exon 47 of the P/Q type calcium channel α1A subunit. These mice overexpress the carboxy terminus (CT) of the P/Q type calcium channel containing an expanded 27 CAG repeat specifically in cerebellar Purkinje cells (CT-longQ27PC). We found that our CT-longQ27PC mice exhibit anxiolytic behavior in the open field, elevated plus maze and light/dark place preference tests, which could be recovered with more threatening conditions such as brighter lighting, meowing sounds and an ultrasound repellent. Their innate fear to find safety in the Barnes maze and visual cliff tests was also diminished with subsequent trials, which could be partially recovered with an ultrasound repellent in the Barnes maze. However, under higher threat conditions such as in the light/dark place preference with ultrasound repellent and in the looming tests, CT-longQ27PC mice responded with higher defensive escape behaviors as controls. Moreover, CT-longQ27PC mice displayed increased levels of CT-labeled aggregates compared with controls. Together these data suggest that cerebellar degeneration by overexpression of CT-longQ27PC is sufficient to impair defensive escape responses in those mice.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures






Similar articles
-
Loss of Flocculus Purkinje Cell Firing Precision Leads to Impaired Gaze Stabilization in a Mouse Model of Spinocerebellar Ataxia Type 6 (SCA6).Cells. 2022 Sep 2;11(17):2739. doi: 10.3390/cells11172739. Cells. 2022. PMID: 36078147 Free PMC article.
-
Spinocerebellar ataxia type 6 protein aggregates cause deficits in motor learning and cerebellar plasticity.J Neurosci. 2015 Jun 10;35(23):8882-95. doi: 10.1523/JNEUROSCI.0891-15.2015. J Neurosci. 2015. PMID: 26063920 Free PMC article.
-
Rapid Onset of Motor Deficits in a Mouse Model of Spinocerebellar Ataxia Type 6 Precedes Late Cerebellar Degeneration.eNeuro. 2015 Dec 26;2(6):ENEURO.0094-15.2015. doi: 10.1523/ENEURO.0094-15.2015. eCollection 2015 Nov-Dec. eNeuro. 2015. PMID: 26730403 Free PMC article.
-
Molecular pathogenesis of spinocerebellar ataxia type 6.Neurotherapeutics. 2007 Apr;4(2):285-94. doi: 10.1016/j.nurt.2007.01.003. Neurotherapeutics. 2007. PMID: 17395139 Review.
-
Spinocerebellar [corrected] Ataxia Type 6: Molecular Mechanisms and Calcium Channel Genetics.Adv Exp Med Biol. 2018;1049:147-173. doi: 10.1007/978-3-319-71779-1_7. Adv Exp Med Biol. 2018. PMID: 29427102 Review.
Cited by
-
Cerebellar contribution to the regulation of defensive states.Front Syst Neurosci. 2023 Mar 31;17:1160083. doi: 10.3389/fnsys.2023.1160083. eCollection 2023. Front Syst Neurosci. 2023. PMID: 37064160 Free PMC article. Review.
-
Memory decline, anxiety and depression in the mouse model of spinocerebellar ataxia type 3.Hum Mol Genet. 2024 Feb 1;33(4):299-317. doi: 10.1093/hmg/ddad179. Hum Mol Genet. 2024. PMID: 37862125 Free PMC article.
-
A combination of chlorzoxazone and folic acid improves recognition memory, anxiety and depression in SCA3-84Q mice.Hum Mol Genet. 2024 Aug 6;33(16):1406-1419. doi: 10.1093/hmg/ddae079. Hum Mol Genet. 2024. PMID: 38727562 Free PMC article.
-
The cerebellum and anxiety.Front Cell Neurosci. 2023 Feb 22;17:1130505. doi: 10.3389/fncel.2023.1130505. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36909285 Free PMC article.
-
Mild Deficits in Fear Learning: Evidence from Humans and Mice with Cerebellar Cortical Degeneration.eNeuro. 2024 Feb 26;11(2):ENEURO.0365-23.2023. doi: 10.1523/ENEURO.0365-23.2023. Print 2024 Feb. eNeuro. 2024. PMID: 38176906 Free PMC article.
References
-
- Laing, P.A.F. and Harrison, B.J. (2021) Safety learning and the Pavlovian conditioned inhibition of fear in humans: current state and future directions. Neurosci. Biobehav. Rev., 127, 659–674. - PubMed
-
- Bentz, D. and Schiller, D. (2015) Threat processing: models and mechanisms. Wiley Interdiscip. Rev. Cogn. Sci., 6, 427–439. - PubMed
-
- Clark, S. (1939) Responses following electrical stimulation of the cerebellar cortex in the normal cat. J. Neurophysiol., 2, 19–36.
-
- Chambers, W.J. (1947) Electrical stimulation of the interior of the cerebellum in the cat. Am. J. Anat., 80, 55–94. - PubMed

