Effectiveness of a COVID-19 Testing Outreach Intervention for Latinx Communities: A Cluster Randomized Trial
- PMID: 35708690
- PMCID: PMC9204550
- DOI: 10.1001/jamanetworkopen.2022.16796
Effectiveness of a COVID-19 Testing Outreach Intervention for Latinx Communities: A Cluster Randomized Trial
Abstract
Importance: Latinx individuals have been disproportionately affected during the COVID-19 pandemic caused by the spread of SARS-CoV-2. It is imperative to evaluate newly developed preventive interventions to assess their effect on COVID-19 health disparities.
Objective: To examine the effectiveness of a culturally tailored outreach intervention designed to increase SARS-CoV-2 testing rates among Latinx populations.
Design, setting, and participants: In this cluster randomized trial performed from February 1 to August 31, 2021, in community settings in 9 Oregon counties, 38 sites were randomized a priori (19 to the community health promoters intervention and 19 to outreach as usual wait-listed controls). Thirty-three sites were activated. A total of 394 SARS-CoV-2 testing events were held and 1851 diagnostic samples collected, of which 919 were from Latinx persons.
Interventions: A culturally informed outreach program was developed that made use of promotores de salud (community health promoters) to increase Latinx SARS-CoV-2 testing. Strategies addressed barriers by disseminating information on testing events in English and Spanish, mitigating misinformation, and increasing trust.
Main outcomes and measures: The primary outcomes were the count of sample tests from Latinx persons and the sampled proportion of the Latinx populace. Site-level covariates included census tract Latinx populace, nativity (number of US-born individuals per 100 population), median age, and income inequality. Time-varying covariates included number of new weekly SARS-CoV-2-positive cases and percentage of vaccine coverage at the county level.
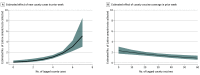
Results: A total of 15 clusters (sites) were randomized to the control group and 18 to the community health promoters group. A total of 1851 test samples were collected, of which 995 (53.8%) were from female participants and 919 (49.6%) were from Latinx individuals. The intervention tested 3.84 (95% CI, 2.47-5.97) times more Latinx individuals per event than controls (incident rate ratio, 0.79; 95% CI, 0.46-1.34; Cohen d = 0.74; P < .001). The intervention was associated with a 0.28 increase in the proportion of Latinx populace being tested compared with control sites for the dependent variable scaled as the proportion of the Latinx populace ×100, or a 0.003 proportion of the raw populace count. The use of a standardized scaling of the proportion of Latinx individuals showed that the relative percentage increase was 0.53 (95% CI, 0.21-0.86) in the intervention sites compared with controls, representing a medium effect size.
Conclusions and relevance: To our knowledge, this was the first randomized evaluation of an outreach intervention designed to increase SARS-CoV-2 testing among Latinx populations. Findings could be used to implement strategies to reduce other health disparities experienced by these groups.
Trial registration: ClinicalTrials.gov Identifier: NCT04793464.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

