JAML promotes acute kidney injury mainly through a macrophage-dependent mechanism
- PMID: 35708906
- PMCID: PMC9431718
- DOI: 10.1172/jci.insight.158571
JAML promotes acute kidney injury mainly through a macrophage-dependent mechanism
Abstract
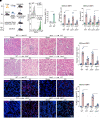
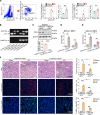
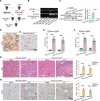
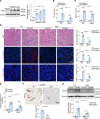
Although macrophages are undoubtedly attractive therapeutic targets for acute kidney injury (AKI) because of their critical roles in renal inflammation and repair, the underlying mechanisms of macrophage phenotype switching and efferocytosis in the regulation of inflammatory responses during AKI are still largely unclear. The present study elucidated the role of junctional adhesion molecule-like protein (JAML) in the pathogenesis of AKI. We found that JAML was significantly upregulated in kidneys from 2 different murine AKI models including renal ischemia/reperfusion injury (IRI) and cisplatin-induced AKI. By generation of bone marrow chimeric mice, macrophage-specific and tubular cell-specific Jaml conditional knockout mice, we demonstrated JAML promoted AKI mainly via a macrophage-dependent mechanism and found that JAML-mediated macrophage phenotype polarization and efferocytosis is one of the critical signal transduction pathways linking inflammatory responses to AKI. Mechanistically, the effects of JAML on the regulation of macrophages were, at least in part, associated with a macrophage-inducible C-type lectin-dependent mechanism. Collectively, our studies explore for the first time to our knowledge new biological functions of JAML in macrophages and conclude that JAML is an important mediator and biomarker of AKI. Pharmacological targeting of JAML-mediated signaling pathways at multiple levels may provide a novel therapeutic strategy for patients with AKI.
Keywords: Macrophages; Nephrology.
Conflict of interest statement
Figures