Novel Budesonide Suppository and Standard Budesonide Rectal Foam Induce High Rates of Clinical Remission and Mucosal Healing in Active Ulcerative Proctitis: a Randomised, Controlled, Non-inferiority Trial
- PMID: 35709376
- PMCID: PMC9683080
- DOI: 10.1093/ecco-jcc/jjac081
Novel Budesonide Suppository and Standard Budesonide Rectal Foam Induce High Rates of Clinical Remission and Mucosal Healing in Active Ulcerative Proctitis: a Randomised, Controlled, Non-inferiority Trial
Abstract
Background and aims: Proctitis is the least extensive type of ulcerative colitis, for which rectal therapy is rarely studied and is underused. This study evaluated the efficacy, safety, and patient's preference of a novel formulation of budesonide suppository 4 mg, compared with a commercially available budesonide rectal foam 2 mg, for the treatment of mild to moderate ulcerative proctitis.
Methods: This was a randomised, double-blind, double-dummy, active-controlled trial. Patients were randomly assigned in a 1:1 ratio to receive either budesonide 4 mg suppository or budesonide 2 mg foam once daily for 8 weeks. The co-primary endpoints were changes from baseline to Week 8 in clinical symptoms, for which clinical remission was defined as having a modified Ulcerative Colitis-Disease Activity Index [UC-DAI] subscore for stool frequency of 0 or 1 and a subscore for rectal bleeding of 0, and mucosal healing, defined as having a modified UC-DAI subscore for mucosal appearance of 0 or 1. Using a more stringent criterion, we additionally analysed deepened mucosal healing, which was defined as a mucosal appearance subscore of 0. Patient's preference, physician's global assessment, and quality of life were also assessed and analysed.
Results: Overall, 286 and 291 patients were included in the 4 mg suppository and 2 mg foam groups, respectively. Budesonide 4 mg suppository met the prespecified criterion for non-inferiority to the 2 mg foam in both co-primary endpoints of clinical remission and mucosal healing. Secondary endpoints consistently supported the non-inferiority of the suppository. Trends in favour of the suppository were observed in the subgroup of mesalazine non-responders. More patients reported a preference for the suppository over rectal foam.
Conclusions: In patients with ulcerative proctitis, budesonide 4 mg suppository was non-inferior to budesonide 2 mg foam in efficacy, and both were safe and well tolerated.
Keywords: Clinical trials; budesonide; rectal foam; rectal suppository; ulcerative proctitis.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Figures

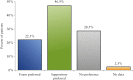
References
-
- Langholz E, Munkholm P, Nielsen OH, Kreiner S, Binder V.. Incidence and prevalence of ulcerative colitis in Copenhagen county from 1962 to 1987. Scand J Gastroenterol 1991;26:1247–56. - PubMed
-
- Langholz E, Munkholm P, Davidsen M, Nielsen OH, Binder V.. Changes in extent of ulcerative colitis: A study on the course and prognostic factors. Scand J Gastroenterol 1996;31:260–6. - PubMed
-
- Harbord M, Eliakim R, Bettenworth D, et al. . Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J Crohns Colitis 2017;11:769–84. - PubMed
-
- Marshall JK, Thabane M, Steinhart AH, et al. . Rectal 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2010:CD004115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

