Convergent Clonal Evolution of Signaling Gene Mutations Is a Hallmark of Myelodysplastic Syndrome Progression
- PMID: 35709710
- PMCID: PMC9338759
- DOI: 10.1158/2643-3230.BCD-21-0155
Convergent Clonal Evolution of Signaling Gene Mutations Is a Hallmark of Myelodysplastic Syndrome Progression
Abstract
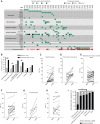
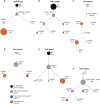
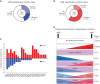
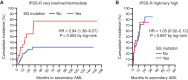
Progression from myelodysplastic syndromes (MDS) to secondary acute myeloid leukemia (AML) is associated with the acquisition and expansion of subclones. Our understanding of subclone evolution during progression, including the frequency and preferred order of gene mutation acquisition, remains incomplete. Sequencing of 43 paired MDS and secondary AML samples identified at least one signaling gene mutation in 44% of MDS and 60% of secondary AML samples, often below the level of standard sequencing detection. In addition, 19% of MDS and 47% of secondary AML patients harbored more than one signaling gene mutation, almost always in separate, coexisting subclones. Signaling gene mutations demonstrated diverse patterns of clonal evolution during disease progression, including acquisition, expansion, persistence, and loss of mutations, with multiple patterns often coexisting in the same patient. Multivariate analysis revealed that MDS patients who had a signaling gene mutation had a higher risk of AML progression, potentially providing a biomarker for progression.
Significance: Subclone expansion is a hallmark of progression from MDS to secondary AML. Subclonal signaling gene mutations are common at MDS (often at low levels), show complex and convergent patterns of clonal evolution, and are associated with future progression to secondary AML. See related article by Guess et al., p. 316 (33). See related commentary by Romine and van Galen, p. 270. This article is highlighted in the In This Issue feature, p. 265.
©2022 American Association for Cancer Research.
Figures


Comment in
-
Rise of the Clones: Myelodysplastic Syndrome to Secondary Acute Myeloid Leukemia.Blood Cancer Discov. 2022 Jul 6;3(4):270-272. doi: 10.1158/2643-3230.BCD-22-0046. Blood Cancer Discov. 2022. PMID: 35709709 Free PMC article.
References
-
- Nimer SD. Myelodysplastic syndromes. Blood 2008;111:4841–51. - PubMed
-
- Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues. WHO Classification of Tumours 2008;22008:439. - PubMed
-
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med 2009;361:1872–85. - PubMed
-
- Granfeldt Ostgard LS, Medeiros BC, Sengelov H, Norgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol 2015;33:3641–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

