The cellular architecture and molecular determinants of the zebrafish fusogenic synapse
- PMID: 35709765
- PMCID: PMC10180866
- DOI: 10.1016/j.devcel.2022.05.016
The cellular architecture and molecular determinants of the zebrafish fusogenic synapse
Abstract
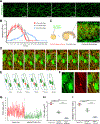
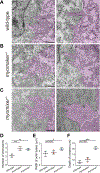
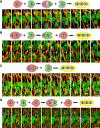
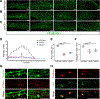
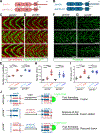
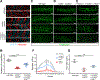
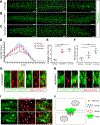
Myoblast fusion is an indispensable process in skeletal muscle development and regeneration. Studies in Drosophila led to the discovery of the asymmetric fusogenic synapse, in which one cell invades its fusion partner with actin-propelled membrane protrusions to promote fusion. However, the timing and sites of vertebrate myoblast fusion remain elusive. Here, we show that fusion between zebrafish fast muscle cells is mediated by an F-actin-enriched invasive structure. Two cell adhesion molecules, Jam2a and Jam3b, are associated with the actin structure, with Jam2a being the major organizer. The Arp2/3 actin nucleation-promoting factors, WAVE and WASP-but not the bipartite fusogenic proteins, Myomaker or Myomixer-promote the formation of the invasive structure. Moreover, the convergence of fusogen-containing microdomains and the invasive protrusions is a prerequisite for cell membrane fusion. Thus, our study provides unprecedented insights into the cellular architecture and molecular determinants of the asymmetric fusogenic synapse in an intact vertebrate animal.
Keywords: Jam2a/Jamb; Jam3b/Jamc; Myomaker; Myomixer; WASP and WAVE; actin cytoskeleton; cell-cell fusion; invasive membrane protrusions; myoblast fusion; zebrafish muscle development.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Similar articles
-
Genetic Mutations in jamb, jamc, and myomaker Revealed Different Roles on Myoblast Fusion and Muscle Growth.Mar Biotechnol (NY). 2019 Feb;21(1):111-123. doi: 10.1007/s10126-018-9865-x. Epub 2018 Nov 22. Mar Biotechnol (NY). 2019. PMID: 30467785 Free PMC article.
-
Requirement of the fusogenic micropeptide myomixer for muscle formation in zebrafish.Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):11950-11955. doi: 10.1073/pnas.1715229114. Epub 2017 Oct 23. Proc Natl Acad Sci U S A. 2017. PMID: 29078404 Free PMC article.
-
The fusogenic synapse at a glance.J Cell Sci. 2019 Sep 16;132(18):jcs213124. doi: 10.1242/jcs.213124. J Cell Sci. 2019. PMID: 31527149 Free PMC article. Review.
-
The regulatory role of Myomaker and Myomixer-Myomerger-Minion in muscle development and regeneration.Cell Mol Life Sci. 2020 Apr;77(8):1551-1569. doi: 10.1007/s00018-019-03341-9. Epub 2019 Oct 23. Cell Mol Life Sci. 2020. PMID: 31642939 Free PMC article. Review.
-
Actin-propelled invasive membrane protrusions promote fusogenic protein engagement during cell-cell fusion.Science. 2013 Apr 19;340(6130):359-63. doi: 10.1126/science.1234781. Epub 2013 Mar 7. Science. 2013. PMID: 23470732 Free PMC article.
Cited by
-
Ectopic expression of Myomaker and Myomixer in slow muscle cells induces slow muscle fusion and myofiber death.J Genet Genomics. 2024 Nov;51(11):1187-1203. doi: 10.1016/j.jgg.2024.08.006. Epub 2024 Aug 30. J Genet Genomics. 2024. PMID: 39209151
-
Molecular regulation of myocyte fusion.Curr Top Dev Biol. 2024;158:53-82. doi: 10.1016/bs.ctdb.2024.01.016. Epub 2024 Mar 16. Curr Top Dev Biol. 2024. PMID: 38670716 Free PMC article. Review.
-
Loss of Myomixer Results in Defective Myoblast Fusion, Impaired Muscle Growth, and Severe Myopathy in Zebrafish.Mar Biotechnol (NY). 2022 Oct;24(5):1023-1038. doi: 10.1007/s10126-022-10159-3. Epub 2022 Sep 9. Mar Biotechnol (NY). 2022. PMID: 36083384 Free PMC article.
-
Novel requirements for HAP2/GCS1-mediated gamete fusion in Tetrahymena.iScience. 2024 May 28;27(6):110146. doi: 10.1016/j.isci.2024.110146. eCollection 2024 Jun 21. iScience. 2024. PMID: 38904066 Free PMC article.
-
Branched actin polymerization drives invasive protrusion formation to promote myoblast fusion during skeletal muscle regeneration.bioRxiv [Preprint]. 2025 Jun 10:2024.09.30.615960. doi: 10.1101/2024.09.30.615960. bioRxiv. 2025. PMID: 39416162 Free PMC article. Preprint.
References
-
- Abmayr SM, Balagopalan L, Galletta BJ, and Hong SJ (2003). Cell and molecular biology of myoblast fusion. Int. Rev. Cytol 225, 33–89. - PubMed
-
- Artero RD, Castanon I, and Baylies MK (2001). The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development 128, 4251–4264. - PubMed
-
- Baas D., Caussanel-Boude S., Guiraud A., Calhabeu F., Delaune E., Pilot F., Chopin E., Machuca-Gayet I., Vernay A., Bertrand S., et al.. (2012). CKIP-1 regulates mammalian and zebrafish myoblast fusion. J. Cell Sci 125, 3790–3800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

