Prospective study of factors associated with asthma attack recurrence (ATTACK) in children from three Ecuadorian cities during COVID-19: a study protocol
- PMID: 35710244
- PMCID: PMC9207574
- DOI: 10.1136/bmjopen-2021-056295
Prospective study of factors associated with asthma attack recurrence (ATTACK) in children from three Ecuadorian cities during COVID-19: a study protocol
Abstract
Introduction: Asthma is a growing health problem in children in marginalised urban settings in low-income and middle-income countries. Asthma attacks are an important cause of emergency care attendance and long-term morbidity. We designed a prospective study, the Asthma Attacks study, to identify factors associated with recurrence of asthma attacks (or exacerbations) among children and adolescents attending emergency care in three Ecuadorian cities.
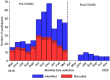
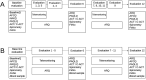
Methods and analysis: Prospective cohort study designed to identify risk factors associated with recurrence of asthma attacks in 450 children and adolescents aged 5-17 years attending emergency care in public hospitals in three Ecuadorian cities (Quito, Cuenca and Portoviejo). The primary outcome will be rate of asthma attack recurrence during up to 12 months of follow-up. Data are being collected at baseline and during follow-up by questionnaire: sociodemographic data, asthma history and management (baseline only); recurrence of asthma symptoms and attacks (monthly); economic costs of asthma to family; Asthma Control Test; Pediatric Asthma Quality of life Questionnaire; and Newcastle Asthma Knowledge Questionnaire (baseline only). In addition, the following are being measured at baseline and during follow-up: lung function and reversibility by spirometry before and after salbutamol; fractional exhaled nitric oxide (FeNO); and presence of IgG antibodies to SARS-CoV-2 in blood. Recruitment started in 2019 but because of severe disruption to emergency services caused by the COVID-19 pandemic, eligibility criteria were modified to include asthmatic children with uncontrolled symptoms and registered with collaborating hospitals. Data will be analysed using logistic regression and survival analyses.
Ethics and dissemination: Ethical approval was obtained from the Hospital General Docente de Calderon (CEISH-HGDC 2019-001) and Ecuadorian Ministry of Public Health (MSP-CGDES-2021-0041-O N° 096-2021). The study results will be disseminated through presentations at conferences and to key stakeholder groups including policy-makers, postgraduate theses, peer-review publications and a study website. Participants gave informed consent to participate in the study before taking part.
Keywords: COVID-19; asthma; epidemiology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Predictors of severe asthma attack re-attendance in Ecuadorian children: a cohort study.Eur Respir J. 2019 Nov 14;54(5):1802419. doi: 10.1183/13993003.02419-2018. Print 2019 Nov. Eur Respir J. 2019. PMID: 31515399 Free PMC article.
-
Health workers' perspectives on asthma care coordination between primary and specialised healthcare in the COVID-19 pandemic: a protocol for a qualitative study in Ecuador and Brazil.BMJ Open. 2021 Nov 1;11(11):e052971. doi: 10.1136/bmjopen-2021-052971. BMJ Open. 2021. PMID: 34725080 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Pediatric pulmonology 2022 year in review: Asthma.Pediatr Pulmonol. 2023 Nov;58(11):3032-3037. doi: 10.1002/ppul.26654. Epub 2023 Aug 29. Pediatr Pulmonol. 2023. PMID: 37642280 Review.
-
Pediatric asthma exacerbation and COVID-19 pandemic: Impacts, challenges, and future considerations.J Asthma. 2024 Feb;61(2):81-91. doi: 10.1080/02770903.2023.2251062. Epub 2023 Sep 1. J Asthma. 2024. PMID: 37610180 Review.
Cited by
-
Ecuadorian healthcare professionals' perspectives on attributes of asthma care coordination: a qualitative study.BMJ Open. 2024 Dec 3;14(12):e084803. doi: 10.1136/bmjopen-2024-084803. BMJ Open. 2024. PMID: 39627140 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
