Current therapies and new developments in NASH
- PMID: 35710299
- PMCID: PMC9484366
- DOI: 10.1136/gutjnl-2021-326874
Current therapies and new developments in NASH
Abstract
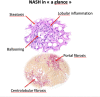
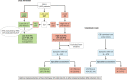
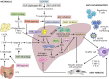
Non-alcoholic steatohepatitis is becoming the most important aetiology for advanced liver disease. There has been important progress in the field in recent years and the complexity of the pathophysiology of NASH is better understood. Multiple non-invasive circulating and imaging biomarkers have been tested. The importance of lifestyle has been recognised and several drugs are being tested in clinical trials. This review addresses the challenges that healthcare professionals face in the management of NASH patients.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: J-FD: Advisory committees : Abbvie, Alientis, Allergan, Axcella, Bayer, Bristol-Myers Squibb, Eisai, Enyo Pharma, Falk, Genfit, Gilead Sciences, HepaRegeniX, Intercept, Ipsen, Inventiva, Madrigal, Merck, Novartis, Pfizer, Roche, Trilliome DTx. Speaking and teaching : Bayer, Intercept, Genfit. Royalties : BMJ Journals, Springer, UpToDate. Quentin Anstee: Research Grant Funding: Abbvie, Allergan/Tobira, AstraZeneca, GlaxoSmithKline, GlympseBio, Novartis Pharma AG, Pfizer. Active Research Collaborations (including research supported through the EU IMI2 LITMUS Consortium*). Abbvie, Antaros Medical*, Allergan/Tobira, AstraZeneca, Boehringer Ingelheim International*, Ellegaard Gottingen Minipigs AS*, Eli Lilly & Company*, Exalenz Bioscience*, Genfit SA*, GlaxoSmithKline, HistoIndex, Intercept Pharma Europe*, iXscient*, Nordic Bioscience*, Novartis Pharma AG*, Novo Nordisk A/S*, One Way Liver Genomics SL*, Perspectum Diagnostics*, Pfizer.*, Sanofi-Aventis Deutschland*, SomaLogic*, Takeda Pharmaceuticals International SA*. Consultancy (undertaken on behalf of Newcastle University): Abbott Laboratories, Acuitas Medical, Allergan/Tobira, E3Bio, EcoR1, Eli Lilly & Company, Galmed, Genfit SA, Gilead, Grunthal, HistoIndex, Imperial Innovations, Intercept Pharma Europe, Inventiva, IQVIA, Janssen, Madrigal, MedImmune, NewGene, NGMBio, Novartis, Novo Nordisk A/S, Pfizer, Poxel, Raptor Pharma, Servier, Viking. Speaker: Abbott Laboratories, Allergan/Tobira, BMS, Clinical Care Options, Falk, Genfit SA, Gilead, Kenes. Elisabetta Bugianesi: Consultant: Gilead Sciences, Intercept, BMS, NovoNordisk, Pfeizer, Inventiva, Genfit, Lilly, MSD. Stephen Harrison: Scientific advisor or consultant for Akero, Alentis, Altimmune, Arrowhead, Axcella, Cirius, Cymabay, Echosens, Fibronostics, Forest Labs, Galectin, Genfit, Gilead,Hepagene, Hepion, HistoIndex, Intercept, Madrigal, Medpace, Metacrine, NGM Bio, Northsea, Novo Nordisk, PathAI, Poxel, Sagimet, Terns, Viking, 89 Bio. Stock options: Akero, Cirius, Galectin, Genfit, Hepion, HistoIndex, PathAI, Metacrine, NGM Bio, Northsea. Grant/Research support: Akero, Axcella, BMS, Cirius, CiVi Biopharma, Conatus, Cymabay, Enyo, Galectin, Genentech, Genfit, Gilead, Hepion, Hightide, Intercept, Madrigal, Metacrine, NGM Bio, Novartis, Novo Nordisk, Northsea, Pfizer, Sagimet, Viking. Rohit Loomba: Research supported by PI, R01DK106419, NIDDK, NIH; PI, R01DK121378, NIDDK, NIH; PI, R01 DK124318, NIDDK, NIH; PI, U01, NASH-CRN, NIDDK, NIH; PI, U01, AA029019, NIAAA, NIH; Project PI, P01HL147835, NHLBI; Investigator Initiated Research Grant, Astrazeneca; Investigator initiated Research Grant, Gilead Inc. Investigator initiated Research grant, Janssen Inc. Valérie Paradis: Consultant: Inventiva; Teaching: Novartis. Vincent Wong: Consultancy: 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Echosens, Gilead Sciences, Inventiva, Merck, Novartis, Novo Nordisk, Pfizer, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, Terns. Lectures: Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk. Research grants: Gilead Sciences Stock: Co-founder of Illuminatio Medical Technology.
Figures

References
-
- Dufour JF, Scherer R, Balp M-M. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors– a targeted literature review. Endocrine and Metabolic Science 2021;3:100089.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
