Ketone body 3-hydroxybutyrate enhances adipocyte function
- PMID: 35710581
- PMCID: PMC9203800
- DOI: 10.1038/s41598-022-14268-w
Ketone body 3-hydroxybutyrate enhances adipocyte function
Abstract
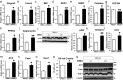
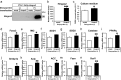
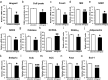
Ketone bodies, including 3HBA, are endogenous products of fatty acid oxidation, and Hmgcs2 is the first rate-limiting enzyme of ketogenesis. From database analysis and in vivo and in vitro experiments, we found that adipose tissue and adipocytes express Hmgcs2, and that adipocytes produce and secrete 3HBA. Treatment with 3HBA enhanced the gene expression levels of the antioxidative stress factors, PPARγ, and lipogenic factors in adipose tissue in vivo and in adipocytes in vitro, accompanied by reduced ROS levels. Knockdown of endogenous Hmgcs2 in adipocytes markedly decreased 3HBA levels in adipocytes and decreased the gene expression levels of the antioxidative stress factors, PPARγ, and lipogenic factors with increased ROS levels. Conversely, overexpression of Hmgcs2 in adipocytes increased 3HBA secretion from adipocytes and enhanced the gene expression levels of the antioxidative stress factors, PPARγ, and lipogenic factors. These results demonstrate that 3HBA plays significant roles in enhancing the physiological function of adipocytes.
© 2022. The Author(s).
Conflict of interest statement
S.N. belongs to the endowed department by KUBARA HONKE Co., Ltd., and A.F. belongs to the endowed department by Takeda Pharmaceutical Company, Rohto Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., FUJI OIL HOLDINGS INC., and Kobayashi Pharmaceutical Co., Ltd. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. All other authors have no conflicts to declare.
Figures
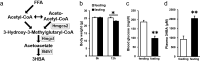




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

