Use of a sutureless aortic valve in reoperative aortic valve replacement
- PMID: 35711205
- PMCID: PMC9196321
- DOI: 10.1016/j.xjtc.2022.02.025
Use of a sutureless aortic valve in reoperative aortic valve replacement
Abstract
Objectives: Management of degenerated bioprosthetic aortic valves remains a challenge. Valve-in-valve transcatheter aortic valve replacement (AVR) has limited utility in the presence of small annuli/prosthetic valves. Sutureless valves may offer an advantage over traditional redo AVR by maximizing effective orifice area due to their unique design as well as ease of implant.
Methods: Twenty-two patients undergoing redo AVR received a sutureless valve in our institution over the past 5 years. All patients were determined to be poor candidates for valve-in-valve transcatheter AVR due to a combination of small annulus size, low coronary heights, and/or underlying valve characteristics (ie, mechanical valves).
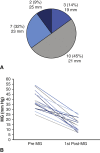
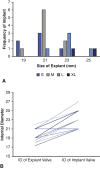
Results: Median time from implant to redo AVR was 8 years. One patient died within 30 days. In the 13 patients who had a 21 mm or smaller valve explanted, 5 small, 7 medium, and 1 large Perceval valves were implanted (all with larger internal diameters than the explanted valve). The average postoperative gradient of the cohort valves was 14.8 mm Hg compared with 38.8 mm Hg preoperatively.
Conclusions: In addition to their ease of use and rapid deployment, sutureless bioprosthetic aortic valves offer significant physiological advantages in patients with degenerated prosthetic aortic valves and small anatomical annuli. It can also simplify the surgical approach to redo AVR following a Bentall procedure. If long-term durability is confirmed, sutureless valves should be considered in a broader population of patients for both redo and primary aortic valve replacement surgery.
Keywords: AI, aortic insufficiency; AVR, aortic valve replacement; PPM, patient–prosthesis mismatch; SAVR; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; TAVR; TAVR, transcatheter aortic valve replacement; ViV TAVR; ViV, valve-in-valve; aortic valve; small annulus.
© 2022 The Author(s).
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

