Immunoproteasome Inhibition Reduces the T Helper 2 Response in Mouse Models of Allergic Airway Inflammation
- PMID: 35711460
- PMCID: PMC9197384
- DOI: 10.3389/fimmu.2022.870720
Immunoproteasome Inhibition Reduces the T Helper 2 Response in Mouse Models of Allergic Airway Inflammation
Abstract
Background: Allergic asthma is a chronic disease and medical treatment often fails to fully control the disease in the long term, leading to a great need for new therapeutic approaches. Immunoproteasome inhibition impairs T helper cell function and is effective in many (auto-) inflammatory settings but its effect on allergic airway inflammation is unknown.
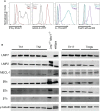
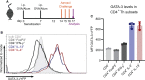
Methods: Immunoproteasome expression was analyzed in in vitro polarized T helper cell subsets. To study Th2 cells in vivo acute allergic airway inflammation was induced in GATIR (GATA-3-vYFP reporter) mice using ovalbumin and house dust mite extract. Mice were treated with the immunoproteasome inhibitor ONX 0914 or vehicle during the challenge phase and the induction of airway inflammation was analyzed.
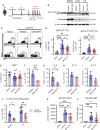
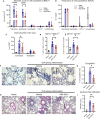
Results: In vitro polarized T helper cell subsets (Th1, Th2, Th17, and Treg) express high levels of immunoproteasome subunits. GATIR mice proved to be a useful tool for identification of Th2 cells. Immunoproteasome inhibition reduced the Th2 response in both airway inflammation models. Furthermore, T cell activation and antigen-specific cytokine secretion was impaired and a reduced infiltration of eosinophils and professional antigen-presenting cells into the lung and the bronchoalveolar space was observed in the ovalbumin model.
Conclusion: These results show the importance of the immunoproteasome in Th2 cells and airway inflammation. Our data provides first insight into the potential of using immunoproteasome inhibition to target the aberrant Th2 response, e.g. in allergic airway inflammation.
Keywords: GATA-3 reporter mice; Th2 cells; allergic airway inflammation; eosinophilia; immunoproteasome inhibition.
Copyright © 2022 Oliveri, Basler, Rao, Fehling and Groettrup.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

