The Underlying Molecular Basis and Mechanisms of Venous Thrombosis in Patients with Osteomyelitis: A Data-Driven Analysis
- PMID: 35711689
- PMCID: PMC9192329
- DOI: 10.1155/2022/5672384
The Underlying Molecular Basis and Mechanisms of Venous Thrombosis in Patients with Osteomyelitis: A Data-Driven Analysis
Abstract
Objective: Osteomyelitis (OM) is one of the most risky and challenging diseases. Emerging evidence indicates OM is a risk factor for increasing incidence of venous thromboembolism (VTE) development. However, the mechanisms have not been intensively investigated.
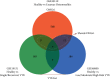
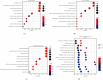
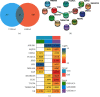
Methods: The OM-related dataset GSE30119 and VTE-related datasets GSE19151 and GSE48000 were downloaded from the Gene Expression Omnibus (GEO) database and analyzed to identify the differentially expressed genes (DEGs) (OMGs1 and VTEGs1, respectively). Functional enrichment analyses of Gene Ontology (GO) terms were performed. VTEGs2 and OMGs2 sharing the common GO biological process (GO-BP) ontology between OMGs1 and VTEGs1 were detected. The TRRUST database was used to identify the upstream transcription factors (TFs) that regulate VTEGs2 and OMGs2. The protein-protein interaction (PPI) network between VTEGs2 and OMGs2 was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING) database and then visualized in Cytoscape. Topological properties of the PPI network were calculated by NetworkAnalyzer. The Molecular Complex Detection (MCODE) plugin was utilized to perform module analysis and choose the hub modules of the PPI network.
Results: A total of 587 OMGs1 and 382 VTEGs1 were identified from the related dataset, respectively. GO-BP terms of OMGs1 and shared DGEs1 were mainly enriched in the neutrophil-related immune response process, and the shared GO-BP terms of OMGs1 and VTEGs1 seemed to be focused on cell activation, immune, defense, and inflammatory response to stress or biotic stimulus. 230 VTEGs2, 333 OMGs2, and 13 shared DEGs2 were detected. 3 TF-target gene pairs (SP1-LSP1, SPI1-FCGR1A, and STAT1-FCGR1A) were identified. The PPI network contained 1611 interactions among 467 nodes. The top 10 hub proteins were TP53, IL4, MPO, ELANE, FOS, CD86, HP, SOCS3, ICAM1, and SNRPG. Several core nodes (such as MPO, ELANE, and CAMP) were essential components of the neutrophil extracellular traps (NETs) network.
Conclusion: This is the first data-mining study to explore shared signatures between OM and VTE by the integrated bioinformatic approach, which can help uncover potential biomarkers and therapeutic targets of OM-related VTE.
Copyright © 2022 Peisheng Chen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

