Synthesis, spectral studies, antioxidant and antibacterial evaluation of aromatic nitro and halogenated tetradentate Schiff bases
- PMID: 35711981
- PMCID: PMC9192811
- DOI: 10.1016/j.heliyon.2022.e09650
Synthesis, spectral studies, antioxidant and antibacterial evaluation of aromatic nitro and halogenated tetradentate Schiff bases
Abstract
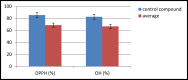
Herein, we report the synthesis, characterization, and biological properties of eleven (3a-3k) novel Schiff bases. The spectral data of FT-IR, 1H NMR, 13C NMR, and LC-MS are associated with these synthesized compounds. From the FT-IR analysis, we confirmed the azomethine (-C=N-) group and from 1H NMR data, the phenolic -OH proton is appeared at range δ 13.92-14.09ppm due to hydrogen bonding. The LC-MS analysis agreed with molecular ion peaks of synthesized Schiff bases. To evaluate the antibacterial activity of newly synthesized compounds were screened against b. licheniformis, b. species, e. coli, and s. aureus. Furthermore, the antioxidant activity was investigated by two methods 2,2-diphenyl-1-picryl hydrazyl (DPPH) and hydroxyl radical scavenging methods. The (-NO2,-Cl,-Br,-I) substituted compounds have shown good antibacterial activity against tested organisms. Also, these compounds were exhibited higher antioxidant activity by given methods.
Keywords: 1-hydroxy-2-acetonapthanone; 1H NMR; Antimicrobial activity; Mass spectrometry; Primary diamines.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis, characterization, spectroscopic studies and biological evaluation of Schiff bases derived from 1-hydroxy-2-acetonapthanone.Heliyon. 2019 Nov 14;5(11):e02774. doi: 10.1016/j.heliyon.2019.e02774. eCollection 2019 Nov. Heliyon. 2019. PMID: 31763472 Free PMC article.
-
Synthesis, Characterization, Antibacterial and Antioxidant Potency of NSubstituted- 2-Sulfanylidene-1,3-Thiazolidin-4-one Derivatives and QSAR Study.Med Chem. 2019;15(8):840-849. doi: 10.2174/1573406415666181205163052. Med Chem. 2019. PMID: 30520381
-
Investigation into the Thermal Response and Pharmacological Activity of Substituted Schiff Bases on α-Amylase and α-Glucosidase.Antioxidants (Basel). 2018 Aug 28;7(9):113. doi: 10.3390/antiox7090113. Antioxidants (Basel). 2018. PMID: 30154344 Free PMC article.
-
Cr(III), Fe(III) and Co(III) complexes of tetradentate (ONNO) Schiff base ligands: synthesis, characterization, properties and biological activity.Spectrochim Acta A Mol Biomol Spectrosc. 2008 Aug;70(3):634-40. doi: 10.1016/j.saa.2007.08.011. Epub 2007 Aug 23. Spectrochim Acta A Mol Biomol Spectrosc. 2008. PMID: 17904895
-
Synthesis, characterization and biological studies of Schiff bases derived from heterocyclic moiety.Bioorg Chem. 2017 Feb;70:67-73. doi: 10.1016/j.bioorg.2016.11.009. Epub 2016 Nov 21. Bioorg Chem. 2017. PMID: 27894775
Cited by
-
Synthesis, Physicochemical Characterization, and Antimicrobial Evaluation of Halogen-Substituted Non-Metal Pyridine Schiff Bases.Molecules. 2024 Oct 6;29(19):4726. doi: 10.3390/molecules29194726. Molecules. 2024. PMID: 39407654 Free PMC article.
-
Comparative study of antioxidant and antimicrobial activity of berberine-derived Schiff bases, nitro-berberine and amino-berberine.Heliyon. 2023 Nov 25;9(12):e22783. doi: 10.1016/j.heliyon.2023.e22783. eCollection 2023 Dec. Heliyon. 2023. PMID: 38058428 Free PMC article.
References
-
- Ejidike I.P., Ajibade P.A. Synthesis and in vitro anticancer, antibacterial, and antioxidant studies of unsymmetrical Schiff base derivatives of 4-[(1E)-N-(2-aminoethyl)ethanimidoyl]benzene-1,3-diol. Res. Chem. Intermed. 2016;42:6543–6555.
-
- Cinarli A., Gürbüz D., Tavman A., Birteksöz A.S. Spectral characterization and antimicrobial activity of some schiff bases derived from 4-chloro-2-aminophenol and various salicylaldehyde derivatives. Chin. J. Chem. 2012;30:449–459.
-
- Beena D. Kumar, Rawat D.S. Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorg. Med. Chem. Lett. 2013;23:641–645. - PubMed
-
- Da Silva C.M., Da Silva D.L., Martins C.V.B., De Resende M.A., Dias E.S., Magalhaes T.F.F., Rodrigues L.P., Sabino A.A., Alves R.B., Fatima A.D. Synthesis of aryl aldimines and their activity against fungi of clinical interest. Chem. Biol. Drug Des. 2011;78:810–815. - PubMed
-
- Gürbüz D., Cinarli A., Tavman A., Birteksöz A.S. Spectral characterization and antimicrobial activity of some schiff bases derived from 4-Methyl-2-aminophenol. Chin. J. Chem. 2012;30:970–978.
LinkOut - more resources
Full Text Sources

