Surface Functionalized MXenes for Wastewater Treatment-A Comprehensive Review
- PMID: 35712023
- PMCID: PMC9189136
- DOI: 10.1002/gch2.202100120
Surface Functionalized MXenes for Wastewater Treatment-A Comprehensive Review
Abstract
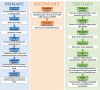
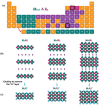
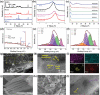
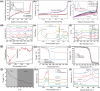
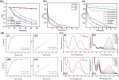
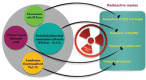
Over 80% of wastewater worldwide is released into the environment without proper treatment. Whilst environmental pollution continues to intensify due to the increase in the number of polluting industries, conventional techniques employed to clean the environment are poorly effective and are expensive. MXenes are a new class of 2D materials that have received a lot of attention for an extensive range of applications due to their tuneable interlayer spacing and tailorable surface chemistry. Several MXene-based nanomaterials with remarkable properties have been proposed, synthesized, and used in environmental remediation applications. In this work, a comprehensive review of the state-of-the-art research progress on the promising potential of surface functionalized MXenes as photocatalysts, adsorbents, and membranes for wastewater treatment is presented. The sources, composition, and effects of wastewater on human health and the environment are displayed. Furthermore, the synthesis, surface functionalization, and characterization techniques of merit used in the study of MXenes are discussed, detailing the effects of a range of factors (e.g., PH, temperature, precursor, etc.) on the synthesis, surface functionalization, and performance of the resulting MXenes. Finally, the limits of MXenes and MXene-based materials as well as their potential future research directions, especially for wastewater treatment applications are highlighted.
Keywords: 2D materials; MXene; functionalization; wastewater treatment.
© 2022 The Authors. Global Challenges published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- World Health Organisation , UN Water, National Systems to Support Drinking‐Water, Sanitation and Hygiene: Global Status Report 2019 , 2019.
-
- Shan Y., Guan D., Liu J., Liu Z., Liu J., Schroeder H., Chen Y., Shao S., Mi Z., Zhang Q., Atmos. Chem. Phys. 2016, 1.
-
- Stocker T. F., Qin D., Plattner G. K., Tignor M. M. B., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P. M., Climate Change 2013. The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 2013.
-
- Ramseur J. L., CRS Report for Congress Deepwater Horizon Oil Spill: The Fate of the Oil, 2010.
-
- Shukla S., Saxena A., in Handbook of Environmental Materials Management (Ed: Hussain C. M.), Springer International Publishing, Cham: 2018, pp. 1–21.
Publication types
LinkOut - more resources
Full Text Sources

