Hyaluronic Acid Hydrogels Hybridized With Au-Triptolide Nanoparticles for Intraarticular Targeted Multi-Therapy of Rheumatoid Arthritis
- PMID: 35712709
- PMCID: PMC9197263
- DOI: 10.3389/fphar.2022.849101
Hyaluronic Acid Hydrogels Hybridized With Au-Triptolide Nanoparticles for Intraarticular Targeted Multi-Therapy of Rheumatoid Arthritis
Abstract
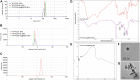
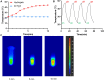
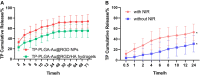
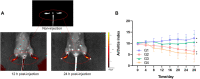
Rheumatoid arthritis (RA) is a chronic inflammatory disease, characterized by synovial inflammation in multiple joints. Triptolide (TP) is a disease-modifying anti-rheumatic drug (DMARD) highly effective in patients with RA and has anti-inflammatory properties. However, its clinical application has been limited owing to practical disadvantages. In the present study, hyaluronic acid (HA) hydrogel-loaded RGD-attached gold nanoparticles (AuNPs) containing TP were synthesized to alleviate the toxicity and increase therapeutic specificity. The hydrogels can be applied for targeted photothermal-chemo treatment and in vivo imaging of RA. Hydrogel systems with tyramine-modified HA (TA-HA) conjugates have been applied to artificial tissue models as surrogates of cartilage to investigate drug transport and release properties. After degradation of HA chains, heat was locally generated at the inflammation region site due to near-infrared resonance (NIR) irradiation of AuNPs, and TP was released from nanoparticles, delivering heat and drug to the inflamed joints simultaneously. RA can be penetrated with NIR light. Intraarticular administration of the hydrogels containing low dosage of TP with NIR irradiation improved the inflamed conditions in mice with collagen-induced arthritis (CIA). Additionally, in vitro experiments were applied to deeply verify the antirheumatic mechanisms of TP-PLGA-Au@RGD/HA hydrogels. TP-PLGA-Au@RGD/HA hydrogel treatment significantly reduced the migratory and invasive capacities of RA fibroblast-like synoviocytes (RA-FLS) in vitro, through the decrease of phosphorylation of mTOR and its substrates, p70S6K1, thus inhibiting the mTOR pathway.
Keywords: NIR imaging; TP-PLGA-Au@RGD/HA hydrogels; mTOR/p70S6K pathway; photothermal-chemo treatment; rheumatoid arthritis.
Copyright © 2022 Li, Liu, Song, Chen, Zhu, Yu, Huang, Zhang, Xue, Hua, Lu, Lu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Arun Kumar R., Sivashanmugam A., Deepthi S., Iseki S., Chennazhi K. P., Nair S. V., et al. (2015). Injectable Chitin-Poly(ε-caprolactone)/Nanohydroxyapatite Composite Microgels Prepared by Simple Regeneration Technique for Bone Tissue Engineering. ACS Appl. Mater. Inter. 7 (18), 9399–9409. 10.1021/acsami.5b02685 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

