Selective Oxidations Using a Cytochrome P450 Enzyme Variant Driven with Surrogate Oxygen Donors and Light
- PMID: 35712785
- PMCID: PMC9541349
- DOI: 10.1002/chem.202201366
Selective Oxidations Using a Cytochrome P450 Enzyme Variant Driven with Surrogate Oxygen Donors and Light
Abstract
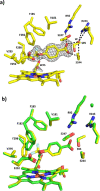
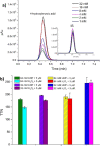
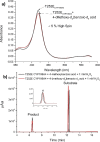
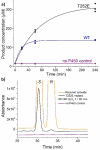
Cytochrome P450 monooxygenase enzymes are versatile catalysts, which have been adapted for multiple applications in chemical synthesis. Mutation of a highly conserved active site threonine to a glutamate can convert these enzymes into peroxygenases that utilise hydrogen peroxide (H2 O2 ). Here, we use the T252E-CYP199A4 variant to study peroxide-driven oxidation activity by using H2 O2 and urea-hydrogen peroxide (UHP). We demonstrate that the T252E variant has a higher stability to H2 O2 in the presence of substrate that can undergo carbon-hydrogen abstraction. This peroxygenase variant could efficiently catalyse O-demethylation and an enantioselective epoxidation reaction (94 % ee). Neither the monooxygenase nor peroxygenase pathways of the P450 demonstrated a significant kinetic isotope effect (KIE) for the oxidation of deuterated substrates. These new peroxygenase variants offer the possibility of simpler cytochrome P450 systems for selective oxidations. To demonstrate this, a light driven H2 O2 generating system was used to support efficient product formation with this peroxygenase enzyme.
Keywords: biocatalysis; heme enzymes; monooxygenases; peroxygenases; protein engineering.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




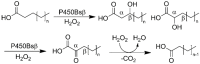


Similar articles
-
Selective carbon-hydrogen bond hydroxylation using an engineered cytochrome P450 peroxygenase.J Inorg Biochem. 2023 Jul;244:112209. doi: 10.1016/j.jinorgbio.2023.112209. Epub 2023 Apr 6. J Inorg Biochem. 2023. PMID: 37080140
-
An In Crystallo Reaction with an Engineered Cytochrome P450 Peroxygenase.Chemistry. 2024 Feb 7;30(8):e202303335. doi: 10.1002/chem.202303335. Epub 2023 Dec 15. Chemistry. 2024. PMID: 37971151
-
Investigating the Active Oxidants Involved in Cytochrome P450 Catalyzed Sulfoxidation Reactions.Chemistry. 2022 Dec 27;28(72):e202202428. doi: 10.1002/chem.202202428. Epub 2022 Nov 9. Chemistry. 2022. PMID: 36169207 Free PMC article.
-
Peroxygenase-catalysed oxyfunctionalisation reactions.Methods Enzymol. 2025;714:425-443. doi: 10.1016/bs.mie.2025.01.041. Epub 2025 Feb 6. Methods Enzymol. 2025. PMID: 40288850 Review.
-
Structure and function of the cytochrome P450 peroxygenase enzymes.Biochem Soc Trans. 2018 Feb 19;46(1):183-196. doi: 10.1042/BST20170218. Epub 2018 Feb 6. Biochem Soc Trans. 2018. PMID: 29432141 Free PMC article. Review.
Cited by
-
Analysis of critical residues for peroxygenation and improved peroxygenase activity via in situ H2O2 generation in CYP105D18.Front Microbiol. 2023 Dec 11;14:1296202. doi: 10.3389/fmicb.2023.1296202. eCollection 2023. Front Microbiol. 2023. PMID: 38149268 Free PMC article.
References
-
- Ortiz de Montellano P. R., in Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed., Springer International Publishing, Switzerland, 2015.
-
- Roiban G. D., Reetz M. T., Chem. Commun. 2015, 51, 2208–2224. - PubMed
-
- Rittle J., Green M. T., Science 2010, 330, 933–937. - PubMed
-
- Groves J. T., McClusky G. A., J. Am. Chem. Soc. 1976, 98, 859–861.
-
- Hannemann F., Bichet A., Ewen K. M., Bernhardt R., Biochim. Biophys. Acta 2007, 1770, 330–344. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

