Development of and insights from systems pharmacology models of antibody-drug conjugates
- PMID: 35712824
- PMCID: PMC9381915
- DOI: 10.1002/psp4.12833
Development of and insights from systems pharmacology models of antibody-drug conjugates
Abstract
Antibody-drug conjugates (ADCs) have gained traction in the oncology space in the past few decades, with significant progress being made in recent years. Although the use of pharmacometric modeling is well-established in the drug development process, there is an increasing need for a better quantitative biological understanding of the pharmacokinetic and pharmacodynamic relationships of these complex molecules. Quantitative systems pharmacology (QSP) approaches can assist in this endeavor; recent computational QSP models incorporate ADC-specific mechanisms and use data-driven simulations to predict experimental outcomes. Various modeling approaches and platforms have been developed at the in vitro, in vivo, and clinical scales, and can be further integrated to facilitate preclinical to clinical translation. These new tools can help researchers better understand the nature and mechanisms of these targeted therapies to help achieve a more favorable therapeutic window. This review delves into the world of systems pharmacology modeling of ADCs, discussing various modeling efforts in the field thus far.
© 2022 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
V.P.R., K.B., and R.H.A. are or were employees and/or shareholders of AstraZeneca at the time of writing of this manuscript and supervised PhD work of I.L. All other authors declared no competing interests for this work. The authors declared no non‐financial competing interests.
Figures
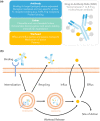


References
-
- Sievers EL, Senter PD. Antibody‐drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15‐29. - PubMed
-
- Polakis P. Antibody drug conjugates for cancer therapy. Pharmacol Rev. 2016;68:3‐19. - PubMed
-
- Shah DK, Haddish‐Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab‐vedotin. J Pharmacokinet Pharmacodyn. 2012;39:643‐659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

