SARS-CoV-2 infection in patients with inborn errors of immunity due to DNA repair defects
- PMID: 35713311
- PMCID: PMC9827799
- DOI: 10.3724/abbs.2022071
SARS-CoV-2 infection in patients with inborn errors of immunity due to DNA repair defects
Abstract
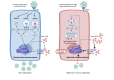
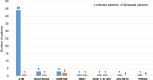
Clinical information on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with inborn errors of immunity (IEI) during the current Coronavirus disease 2019 (COVID-19) pandemic is still limited. Proper DNA repair machinery is required for the development of the adaptive immune system, which provides specific and long-term protection against SARS-CoV-2. This review highlights the impact of SARS-CoV-2 infections on IEI patients with DNA repair disorders and summarizes susceptibility risk factors, pathogenic mechanisms, clinical manifestations and management strategies of COVID-19 in this special patient population.
Keywords: COVID-19; DNA repair mechanism; inborn errors of immunity; primary immunodeficiency.
Conflict of interest statement
The authors declared that they have no conflict of interest.
Figures

Similar articles
-
Coronavirus disease 2019 in patients with inborn errors of immunity: An international study.J Allergy Clin Immunol. 2021 Feb;147(2):520-531. doi: 10.1016/j.jaci.2020.09.010. Epub 2020 Sep 24. J Allergy Clin Immunol. 2021. PMID: 32980424 Free PMC article.
-
Impact of SARS-CoV-2 infection and COVID-19 on patients with inborn errors of immunity.J Allergy Clin Immunol. 2023 Apr;151(4):818-831. doi: 10.1016/j.jaci.2022.11.010. Epub 2022 Dec 13. J Allergy Clin Immunol. 2023. PMID: 36522221 Free PMC article. Review.
-
Coronavirus disease 2019 in patients with inborn errors of immunity: lessons learned.Curr Opin Pediatr. 2021 Dec 1;33(6):648-656. doi: 10.1097/MOP.0000000000001062. Curr Opin Pediatr. 2021. PMID: 34734915 Free PMC article. Review.
-
Outcome of SARS-CoV-2 Infection in 121 Patients with Inborn Errors of Immunity: A Cross-Sectional Study.J Clin Immunol. 2021 Oct;41(7):1479-1489. doi: 10.1007/s10875-021-01066-8. Epub 2021 Jun 23. J Clin Immunol. 2021. PMID: 34164762 Free PMC article.
-
COVID-19 in the Context of Inborn Errors of Immunity: a Case Series of 31 Patients from Mexico.J Clin Immunol. 2021 Oct;41(7):1463-1478. doi: 10.1007/s10875-021-01077-5. Epub 2021 Jun 10. J Clin Immunol. 2021. PMID: 34114122 Free PMC article.
Cited by
-
Severity of SARS-CoV-2 infection in children with inborn errors of immunity (primary immunodeficiencies): a systematic review.Allergy Asthma Clin Immunol. 2023 Aug 9;19(1):69. doi: 10.1186/s13223-023-00831-1. Allergy Asthma Clin Immunol. 2023. PMID: 37559153 Free PMC article.
-
Install, repair and outcomes: a special issue on physiological and programmed DNA lesions.Acta Biochim Biophys Sin (Shanghai). 2022 May 25;54(6):757-758. doi: 10.3724/abbs.2022058. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35713315 Free PMC article. No abstract available.
-
Human genetic and immunological determinants of SARS-CoV-2 infection and multisystem inflammatory syndrome in children.Clin Exp Immunol. 2025 Jan 21;219(1):uxae062. doi: 10.1093/cei/uxae062. Clin Exp Immunol. 2025. PMID: 39028583 Free PMC article. Review.
References
-
- Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. . 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. - DOI - PMC - PubMed
-
- Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S, Wang Q, Yu F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Sig Transduct Target Ther. . 2021;6:233. doi: 10.1038/s41392-021-00653-w. - DOI - PMC - PubMed
-
- V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. . 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. - DOI - PMC - PubMed
-
- Ascough S, Paterson S, Chiu C. Induction and subversion of human protective immunity: contrasting influenza and respiratory syncytial virus. Front Immunol. . 2018;9:323. doi: 10.3389/fimmu.2018.00323. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

