BTDAzo: A Photoswitchable TRPC5 Channel Activator
- PMID: 35713469
- PMCID: PMC9542918
- DOI: 10.1002/anie.202201565
BTDAzo: A Photoswitchable TRPC5 Channel Activator
Abstract
Photoswitchable reagents can be powerful tools for high-precision biological control. TRPC5 is a Ca2+ -permeable cation channel with distinct tissue-specific roles, from synaptic function to hormone regulation. Reagents giving spatiotemporally-resolved control over TRPC5 activity may be key to understanding and harnessing its biology. Here we develop the first photoswitchable TRPC5-modulator, BTDAzo, to address this goal. BTDAzo can photocontrol TRPC5 currents in cell culture, as well as controlling endogenous TRPC5-based neuronal Ca2+ responses in mouse brain slices. BTDAzos are also the first reported azo-benzothiadiazines, an accessible and conveniently derivatised azoheteroarene with strong two-colour photoswitching. BTDAzo's ability to control TRPC5 across relevant channel biology settings makes it suitable for a range of dynamically reversible photoswitching studies in TRP channel biology, with the aim to decipher the various biological roles of this centrally important ion channel.
Keywords: Agonists; Cation Channels; Ligand Design; Photopharmacology; Prolactin Signaling.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
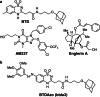
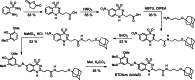



References
-
- “Mammalian Transient Receptor Potential (TRP) Cation Channels”: Flockerzi V., Nilius B. in Handbook of Experimental Pharmacology, Vol. 222 (Eds.: Nilius B., Flockerzi V.), Springer, Berlin, 2014, pp. 1–12. - PubMed
-
- Sharma S., Hopkins C. R., J. Med. Chem. 2019, 62, 7589–7602. - PubMed
-
- Curcic S., Tiapko O., Groschner K., Pharmacol. Ther. 2019, 200, 13–26. - PubMed
-
- Leinders-Zufall T., Storch U., Bleymehl K., Mederos y Schnitzler M., Frank J. A., Konrad D. B., Trauner D., Gudermann T., Zufall F., Cell Chem. Biol. 2018, 25, 215–223.e3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

