Soluble RAGE Prevents Type 1 Diabetes Expanding Functional Regulatory T Cells
- PMID: 35713929
- PMCID: PMC9862506
- DOI: 10.2337/db22-0177
Soluble RAGE Prevents Type 1 Diabetes Expanding Functional Regulatory T Cells
Abstract
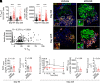
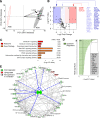
Type 1 diabetes is an autoimmune disease with no cure, where clinical translation of promising therapeutics has been hampered by the reproducibility crisis. Here, short-term administration of an antagonist to the receptor for advanced glycation end products (sRAGE) protected against murine diabetes at two independent research centers. Treatment with sRAGE increased regulatory T cells (Tregs) within the islets, pancreatic lymph nodes, and spleen, increasing islet insulin expression and function. Diabetes protection was abrogated by Treg depletion and shown to be dependent on antagonizing RAGE with use of knockout mice. Human Tregs treated with a RAGE ligand downregulated genes for suppression, migration, and Treg homeostasis (FOXP3, IL7R, TIGIT, JAK1, STAT3, STAT5b, CCR4). Loss of suppressive function was reversed by sRAGE, where Tregs increased proliferation and suppressed conventional T-cell division, confirming that sRAGE expands functional human Tregs. These results highlight sRAGE as an attractive treatment to prevent diabetes, showing efficacy and reproducibility at multiple research centers and in human T cells.
Trial registration: ClinicalTrials.gov NCT01030861 NCT01773707.
© 2022 by the American Diabetes Association.
Conflict of interest statement
Figures




References
-
- Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes 2005;54:1407–1414 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

