Host cell-dependent late entry step as determinant of hepatitis B virus infection
- PMID: 35714170
- PMCID: PMC9246237
- DOI: 10.1371/journal.ppat.1010633
Host cell-dependent late entry step as determinant of hepatitis B virus infection
Abstract
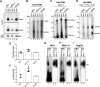
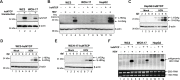
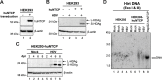
Hepatitis B virus (HBV) has a highly restricted host range and cell tropism. Other than the human sodium taurocholate cotransporting polypeptide (huNTCP), the HBV entry receptor, host determinants of HBV susceptibility are poorly understood. Woodchucks are naturally infected with woodchuck hepatitis virus (WHV), closely related to HBV, but not with HBV. Here, we investigated the capabilities of woodchuck hepatic and human non-hepatic cell lines to support HBV infection. DNA transfection assays indicated that all cells tested supported both HBV and WHV replication steps post entry, including the viral covalently closed circular DNA (cccDNA) formation, which is essential for establishing and sustaining infection. Ectopic expression of huNTCP rendered one, but not the other, woodchuck hepatic cell line and the non-hepatic human cell line competent to support productive HBV entry, defined here by cccDNA formation during de novo infection. All huNTCP-expressing cell lines tested became susceptible to infection with hepatitis D virus (HDV) that shares the same entry receptor and initial steps of entry with HBV, suggesting that a late entry/trafficking step(s) of HBV infection was defective in one of the two woodchuck cell lines. In addition, the non-susceptible woodchuck hepatic cell line became susceptible to HBV after fusion with human hepatic cells, suggesting the lack of a host cell-dependent factor(s) in these cells. Comparative transcriptomic analysis of the two woodchuck cell lines revealed widespread differences in gene expression in multiple biological processes that may contribute to HBV infection. In conclusion, other than huNTCP, neither human- nor hepatocyte-specific factors are essential for productive HBV entry. Furthermore, a late trafficking step(s) during HBV infection, following the shared entry steps with HDV and before cccDNA formation, is subject to host cell regulation and thus, a host determinant of HBV infection.
Conflict of interest statement
The authors have declared that no competing interests exists.
Figures
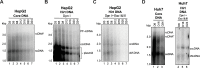

Similar articles
-
Aborted infection of human sodium taurocholate cotransporting polypeptide (hNTCP) expressing woodchuck hepatocytes with hepatitis B virus (HBV).Virus Genes. 2023 Dec;59(6):823-830. doi: 10.1007/s11262-023-02031-w. Epub 2023 Sep 20. Virus Genes. 2023. PMID: 37728707
-
Replication of naturally occurring woodchuck hepatitis virus deletion mutants in primary hepatocyte cultures and after transmission to naive woodchucks.J Virol. 2001 Apr;75(8):3811-8. doi: 10.1128/JVI.75.8.3811-3818.2001. J Virol. 2001. PMID: 11264370 Free PMC article.
-
Limited disassembly of cytoplasmic hepatitis B virus nucleocapsids restricts viral infection in murine hepatic cells.Hepatology. 2023 Apr 1;77(4):1366-1381. doi: 10.1002/hep.32622. Epub 2022 Jul 5. Hepatology. 2023. PMID: 35718932
-
Hepatitis B Virus Capsid: The Core in Productive Entry and Covalently Closed Circular DNA Formation.Viruses. 2023 Feb 28;15(3):642. doi: 10.3390/v15030642. Viruses. 2023. PMID: 36992351 Free PMC article. Review.
-
Diverse Virus and Host-Dependent Mechanisms Influence the Systemic and Intrahepatic Immune Responses in the Woodchuck Model of Hepatitis B.Front Immunol. 2020 May 27;11:853. doi: 10.3389/fimmu.2020.00853. eCollection 2020. Front Immunol. 2020. PMID: 32536912 Free PMC article. Review.
Cited by
-
T Cell Response to SARS-CoV-2 Coinfection and Comorbidities.Pathogens. 2023 Feb 15;12(2):321. doi: 10.3390/pathogens12020321. Pathogens. 2023. PMID: 36839596 Free PMC article. Review.
-
Viral Oncogenesis: Synergistic Role of Genome Integration and Persistence.Viruses. 2024 Dec 23;16(12):1965. doi: 10.3390/v16121965. Viruses. 2024. PMID: 39772271 Free PMC article. Review.
-
Detection of Hepatitis B Virus Covalently Closed Circular DNA and Intermediates in Its Formation.Methods Mol Biol. 2024;2837:99-111. doi: 10.1007/978-1-0716-4027-2_9. Methods Mol Biol. 2024. PMID: 39044078 Free PMC article.
-
Aborted infection of human sodium taurocholate cotransporting polypeptide (hNTCP) expressing woodchuck hepatocytes with hepatitis B virus (HBV).Virus Genes. 2023 Dec;59(6):823-830. doi: 10.1007/s11262-023-02031-w. Epub 2023 Sep 20. Virus Genes. 2023. PMID: 37728707
-
Characterization of Intracellular Precore-Derived Proteins and Their Functions in Hepatitis B Virus-Infected Human Hepatocytes.mBio. 2023 Feb 28;14(1):e0350122. doi: 10.1128/mbio.03501-22. Epub 2023 Jan 30. mBio. 2023. PMID: 36715515 Free PMC article.
References
-
- WHO. Hepatitis B: World Health Organization; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

