Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary
- PMID: 35714185
- PMCID: PMC9205599
- DOI: 10.1126/sciadv.abn4564
Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary
Abstract
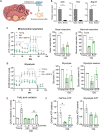
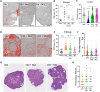
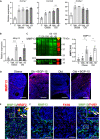
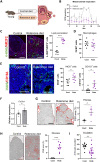
The female ovary contains a finite number of oocytes, and their release at ovulation becomes sporadic and disordered with aging and with obesity, leading to loss of fertility. Understanding the molecular defects underpinning this pathology is essential as age of childbearing and obesity rates increase globally. We identify that fibrosis within the ovarian stromal compartment is an underlying mechanism responsible for impaired oocyte release, which is initiated by mitochondrial dysfunction leading to diminished bioenergetics, oxidative damage, inflammation, and collagen deposition. Furthermore, antifibrosis drugs (pirfenidone and BGP-15) eliminate fibrotic collagen and restore ovulation in reproductively old and obese mice, in association with dampened M2 macrophage polarization and up-regulated MMP13 protease. This is the first evidence that ovarian fibrosis is reversible and indicates that drugs targeting mitochondrial metabolism may be a viable therapeutic strategy for women with metabolic disorders or advancing age to maintain ovarian function and extend fertility.
Figures






References
-
- Heffner L. J., Advanced maternal age—How old is too old? N. Engl. J. Med. 351, 1927–1929 (2004). - PubMed
-
- Broekmans F. J., Soules M. R., Fauser B. C., Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 30, 465–493 (2009). - PubMed
-
- Faddy M. J., Gosden R. G., Gougeon A., Richardson S. J., Nelson J. F., Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 7, 1342–1346 (1992). - PubMed
-
- McCloskey C. W., Cook D. P., Kelly B. S., Azzi F., Allen C. H., Forsyth A., Upham J., Rayner K. J., Gray D. A., Boyd R. W., Murugkar S., Lo B., Trudel D., Senterman M. K., Vanderhyden B. C., Metformin abrogates age-associated ovarian fibrosis. Clin. Cancer Res. 26, 632–642 (2020). - PubMed
-
- Amargant F., Manuel S. L., Tu Q., Parkes W. S., Rivas F., Zhou L. T., Rowley J. E., Villanueva C. E., Hornick J. E., Shekhawat G. S., Wei J. J., Pavone M. E., Hall A. R., Pritchard M. T., Duncan F. E., Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 19, e13259 (2020). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

