The phagocytic cyst cells in Drosophila testis eliminate germ cell progenitors via phagoptosis
- PMID: 35714186
- PMCID: PMC9205596
- DOI: 10.1126/sciadv.abm4937
The phagocytic cyst cells in Drosophila testis eliminate germ cell progenitors via phagoptosis
Abstract
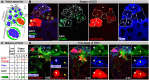
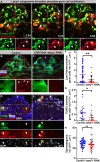
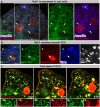
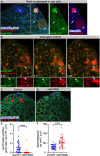
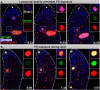
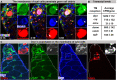
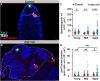
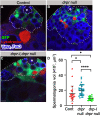
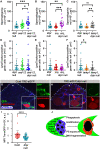
Phagoptosis is a frequently occurring nonautonomous cell death pathway in which phagocytes eliminate viable cells. While it is thought that phosphatidylserine (PS) "eat-me" signals on target cells initiate the process, the precise sequence of events is largely unknown. Here, we show that in Drosophila testes, progenitor germ cells are spontaneously removed by neighboring cyst cells through phagoptosis. Using live imaging with multiple markers, we demonstrate that cyst cell-derived early/late endosomes and lysosomes fused around live progenitors to acidify them, before DNA fragmentation and substantial PS exposure on the germ cell surface. Furthermore, the phagocytic receptor Draper is expressed on cyst cell membranes and is necessary for phagoptosis. Significantly, germ cell death is blocked by knockdown of either the endosomal component Rab5 or the lysosomal associated protein Lamp1, within the cyst cells. These data ascribe an active role for phagocytic cyst cells in removal of live germ cell progenitors.
Figures
References
-
- Galluzzi L., Vitale I., Aaronson S. A., Abrams J. M., Adam D., Agostinis P., Alnemri E. S., Altucci L., Amelio I., Andrews D. W., Annicchiarico-Petruzzelli M., Antonov A. V., Arama E., Baehrecke E. H., Barlev N. A., Bazan N. G., Bernassola F., Bertrand M. J. M., Bianchi K., Blagosklonny M. V., Blomgren K., Borner C., Boya P., Brenner C., Campanella M., Candi E., Carmona-Gutierrez D., Cecconi F., Chan F. K. M., Chandel N. S., Cheng E. H., Chipuk J. E., Cidlowski J. A., Ciechanover A., Cohen G. M., Conrad M., Cubillos-Ruiz J. R., Czabotar P. E., D’Angiolella V., Dawson T. M., Dawson V. L., de Laurenzi V., de Maria R., Debatin K. M., DeBerardinis R. J., Deshmukh M., di Daniele N., di Virgilio F., Dixit V. M., Dixon S. J., Duckett C. S., Dynlacht B. D., el-Deiry W. S., Elrod J. W., Fimia G. M., Fulda S., García-Sáez A. J., Garg A. D., Garrido C., Gavathiotis E., Golstein P., Gottlieb E., Green D. R., Greene L. A., Gronemeyer H., Gross A., Hajnoczky G., Hardwick J. M., Harris I. S., Hengartner M. O., Hetz C., Ichijo H., Jäättelä M., Joseph B., Jost P. J., Juin P. P., Kaiser W. J., Karin M., Kaufmann T., Kepp O., Kimchi A., Kitsis R. N., Klionsky D. J., Knight R. A., Kumar S., Lee S. W., Lemasters J. J., Levine B., Linkermann A., Lipton S. A., Lockshin R. A., López-Otín C., Lowe S. W., Luedde T., Lugli E., MacFarlane M., Madeo F., Malewicz M., Malorni W., Manic G., Marine J. C., Martin S. J., Martinou J. C., Medema J. P., Mehlen P., Meier P., Melino S., Miao E. A., Molkentin J. D., Moll U. M., Muñoz-Pinedo C., Nagata S., Nuñez G., Oberst A., Oren M., Overholtzer M., Pagano M., Panaretakis T., Pasparakis M., Penninger J. M., Pereira D. M., Pervaiz S., Peter M. E., Piacentini M., Pinton P., Prehn J. H. M., Puthalakath H., Rabinovich G. A., Rehm M., Rizzuto R., Rodrigues C. M. P., Rubinsztein D. C., Rudel T., Ryan K. M., Sayan E., Scorrano L., Shao F., Shi Y., Silke J., Simon H. U., Sistigu A., Stockwell B. R., Strasser A., Szabadkai G., Tait S. W. G., Tang D., Tavernarakis N., Thorburn A., Tsujimoto Y., Turk B., vanden Berghe T., Vandenabeele P., Vander Heiden M. G., Villunger A., Virgin H. W., Vousden K. H., Vucic D., Wagner E. F., Walczak H., Wallach D., Wang Y., Wells J. A., Wood W., Yuan J., Zakeri Z., Zhivotovsky B., Zitvogel L., Melino G., Kroemer G., Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25, 486–541 (2018). - PMC - PubMed
-
- Shklover J., Levy-Adam F., Kurant E., Apoptotic cell clearance in development. Curr. Top. Dev. Biol. 114, 297–334 (2015). - PubMed
-
- Brown G. C., Vilalta A., Fricker M., Phagoptosis - Cell death by phagocytosis – Plays central roles in physiology, host defense and pathology. Curr. Mol. Med. 15, 842–851 (2015). - PubMed
-
- Brown G. C., Neher J. J., Eaten alive! Cell death by primary phagocytosis: ‘Phagoptosis’. Trends Biochem. Sci. 37, 325–332 (2012). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

