The oral ferroportin inhibitor vamifeport improves hemodynamics in a mouse model of sickle cell disease
- PMID: 35714304
- PMCID: PMC9389634
- DOI: 10.1182/blood.2021014716
The oral ferroportin inhibitor vamifeport improves hemodynamics in a mouse model of sickle cell disease
Abstract
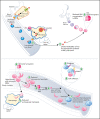
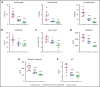
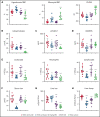
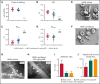
Sickle cell disease (SCD) is an inherited hemolytic anemia caused by a single point mutation in the β-globin gene of hemoglobin that leads to synthesis of sickle hemoglobin (HbS) in red blood cells (RBCs). HbS polymerizes in hypoxic conditions, leading to intravascular hemolysis, release of free hemoglobin and heme, and increased adhesion of blood cells to the endothelial vasculature, which causes painful vaso-occlusion and organ damage. HbS polymerization kinetics are strongly dependent on the intracellular HbS concentration; a relatively small reduction in cellular HbS concentration may prevent HbS polymerization and its sequelae. We hypothesized that iron restriction via blocking ferroportin, the unique iron transporter in mammals, might reduce HbS concentration in RBCs, thereby decreasing hemolysis, improving blood flow, and preventing vaso-occlusive events. Indeed, vamifeport (also known as VIT-2763), a clinical-stage oral ferroportin inhibitor, reduced hemolysis markers in the Townes model of SCD. The RBC indices of vamifeport-treated male and female Townes mice exhibited changes attributable to iron-restricted erythropoiesis: decreased corpuscular hemoglobin concentration mean and mean corpuscular volume, as well as increased hypochromic and microcytic RBC fractions. Furthermore, vamifeport reduced plasma soluble VCAM-1 concentrations, which suggests lowered vascular inflammation. Accordingly, intravital video microscopy of fluorescently labeled blood cells in the microvasculature of Townes mice treated with vamifeport revealed diminished adhesion to the endothelium and improved hemodynamics. These preclinical data provide a strong proof-of-concept for vamifeport in the Townes model of SCD and support further development of this compound as a potential novel therapy in SCD.
© 2022 by The American Society of Hematology.
Figures




Comment in
-
Isn't it ironic: better RBCs by blocking iron.Blood. 2022 Aug 18;140(7):669-670. doi: 10.1182/blood.2022017209. Blood. 2022. PMID: 35980684 Free PMC article. No abstract available.
References
-
- Kato GJ, Piel FB, Reid CD, et al. . Sickle cell disease. Nat Rev Dis Primers. 2018;4(1):18010. - PubMed
-
- Wagener FA, Eggert A, Boerman OC, et al. . Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98(6):1802-1811. - PubMed
-
- Figueiredo RT, Fernandez PL, Mourao-Sa DS, et al. . Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282(28):20221-20229. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

