Real-life data on treatment and outcomes in advanced ovarian cancer: An observational, multinational cohort study (RESPONSE trial)
- PMID: 35714310
- PMCID: PMC9545328
- DOI: 10.1002/cncr.34350
Real-life data on treatment and outcomes in advanced ovarian cancer: An observational, multinational cohort study (RESPONSE trial)
Abstract
Background: This study aimed to describe the treatment strategies and outcomes for women with newly diagnosed advanced high-grade serous or endometrioid ovarian cancer (OC).
Methods: This observational study collected real-world medical record data from eight Western countries on the diagnostic workup, clinical outcomes, and treatment of adult women with newly diagnosed advanced (Stage III-IV) high-grade serous or endometrioid OC. Patients were selected backward in time from April 1, 2018 (the index date), with a target of 120 patients set per country, followed for ≥20 months.
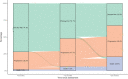
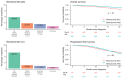
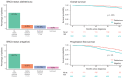
Results: Of the 1119 women included, 66.9% had Stage III disease, 11.7% had a deleterious BRCA mutation, and 26.6% received bevacizumab; 40.8% and 39.3% underwent primary debulking surgery (PDS) and interval debulking surgery (IDS), respectively. Of the patients who underwent PDS, 55.5% had no visible residual disease (VRD); 63.9% of the IDS patients had no VRD. According to physician-assessed responses (at the first assessment after diagnosis and treatment), 53.2% of the total population had a complete response and 25.7% had a partial response to first-line chemotherapy after surgery. After ≥20 months of follow-up, 32.9% of the patients were disease-free, 46.4% had progressive disease, and 20.6% had died. Bevacizumab use had a significant positive effect on overall survival (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91; p = .01). A deleterious BRCA status had a significant positive effect on progression-free survival (HR, 0.60; 95% CI, 0.41-0.84; p < .01).
Conclusions: Women with advanced high-grade serous or endometrioid OC have a poor prognosis. Bevacizumab use and a deleterious BRCA status were found to improve survival in this real-world population.
Lay summary: Patients with advanced (Stage III or IV) ovarian cancer (OC) have a poor prognosis. The standard treatment options of surgery and chemotherapy extend life beyond diagnosis for 5 years or more in only approximately 45% of patients. This study was aimed at describing the standard of care in eight Western countries and estimating how many patients who are diagnosed with high-grade serous or endometrioid OC could potentially be eligible for first-line poly(adenosine diphosphate ribose) polymerase inhibitor (PARPi) maintenance therapy. The results highlight the poor prognosis for these patients and suggest that a significant proportion (79%) would potentially be eligible for first-line PARPi maintenance treatment.
Keywords: bevacizumab; first-line treatment; ovarian cancer; poly(adenosine diphosphate ribose) polymerase (PARP) inhibitor; real-world data.
© 2022 The Authors. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Christian Marth reports consulting fees from Roche, Novartis, Amgen, MSD, AstraZeneca, Pfizer, PharmaMar, Cerulean, Vertex, Tesaro, GSK, and Seagen; honoraria from Roche, Novartis, Amgen, MSD, AstraZeneca, PharmaMar, Tesaro, GSK, and Seagen; support for attending meetings from Roche and AstraZeneca; and participation on data safety monitoring or advisory boards for Roche, Novartis, Amgen, MSD, AstraZeneca, Pfizer, PharmaMar, Cerulean, Vertex, Tesaro, GSK, and Seagen. Klaus Kaae Andersen is an employee of and holds stock in AstraZeneca. Karoliina M. Aro reports payments or honoraria from Gedeon Richter. Maria de Lurdes Batarda reports support for attending meetings and/or travel from AstraZeneca. Anne Weng Ekmann‐Gade has received honoraria for consultation from AstraZeneca (this study). Ulla‐Maija Haltia has received honoraria for consultation from AstraZeneca (this study). Christian Marth has received honoraria for consultation from AstraZeneca (this study). Maria de Lurdes Batarda has received honoraria for consultation from AstraZeneca (this study). Jesper Hansen is an employee of AstraZeneca. Heini Lassus has received honoraria for consultation or lectures from AstraZeneca (this study), GSK, Eisai, and Roche. Kristina Lindemann reports consulting fees from AstraZeneca (paid to her institution); participation on boards for GSK, MSD, and Eisai; and leadership or fiduciary roles with the Nordic Society of Gynaecological Oncology and the Cancer Registry of Norway. Stephan Polterauer reports honoraria for consultation from AstraZeneca, Celgene, GSK, Eisai, MSD, PharmaMar, Roche, Roche Diagnostics, Meda Pharma, Tesaro, and Vifor Pharma; honoraria for lectures from AstraZeneca, Eisai, MSD, GSK, Tesaro, MedAhead, and KLI; and participation on advisory boards for AstraZeneca, Eisai, MSD, GSK, Tesaro, and PharmaMar. The other authors made no disclosures.
Figures
References
-
- Estimates of cancer incidence and mortality in 2020, for all cancer sites . European Cancer Information System. https://ecis.jrc.ec.europa.eu/index.php. Accessed July 6, 2021.
-
- European Institute of Women's Health. Ovarian cancer: a silent killer. Eurohealth. https://eurohealth.ie/policy‐brief‐women‐and‐ovarian‐cancer‐in‐the‐eu‐2018/. Accessed July 6, 2021.
-
- Colombo N, Sessa C, du Bois A, et al. ESMO‐ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

