Enasidenib vs conventional care in older patients with late-stage mutant-IDH2 relapsed/refractory AML: a randomized phase 3 trial
- PMID: 35714312
- PMCID: PMC10644040
- DOI: 10.1182/blood.2021014901
Enasidenib vs conventional care in older patients with late-stage mutant-IDH2 relapsed/refractory AML: a randomized phase 3 trial
Abstract
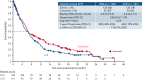
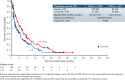
This open-label, randomized, phase 3 trial (NCT02577406) compared enasidenib, an oral IDH2 (isocitrate dehydrogenase 2) inhibitor, with conventional care regimens (CCRs) in patients aged ≥60 years with late-stage, mutant-IDH2 acute myeloid leukemia (AML) relapsed/refractory (R/R) to 2 or 3 prior AML-directed therapies. Patients were first preselected to a CCR (azacitidine, intermediate-dose cytarabine, low-dose cytarabine, or supportive care) and then randomized (1:1) to enasidenib 100 mg per day or CCR. The primary endpoint was overall survival (OS). Secondary endpoints included event-free survival (EFS), time to treatment failure (TTF), overall response rate (ORR), hematologic improvement (HI), and transfusion independence (TI). Overall, 319 patients were randomized to enasidenib (n = 158) or CCR (n = 161). The median age was 71 years, median (range) enasidenib exposure was 142 days (3 to 1270), and CCR was 36 days (1 to 1166). One enasidenib (0.6%) and 20 CCR (12%) patients received no randomized treatment, and 30% and 43%, respectively, received subsequent AML-directed therapies during follow-up. The median OS with enasidenib vs CCR was 6.5 vs 6.2 months (HR [hazard ratio], 0.86; P = .23); 1-year survival was 37.5% vs 26.1%. Enasidenib meaningfully improved EFS (median, 4.9 vs 2.6 months with CCR; HR, 0.68; P = .008), TTF (median, 4.9 vs 1.9 months; HR, 0.53; P < .001), ORR (40.5% vs 9.9%; P <.001), HI (42.4% vs 11.2%), and red blood cell (RBC)-TI (31.7% vs 9.3%). Enasidenib safety was consistent with prior reports. The primary study endpoint was not met, but OS was confounded by early dropout and subsequent AML-directed therapies. Enasidenib provided meaningful benefits in EFS, TTF, ORR, HI, and RBC-TI in this heavily pretreated older mutant-IDH2 R/R AML population.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.dB. reports grants and personal fees from Agios and Forma; personal fees from AbbVie, Astellas, Celgene, Daiichi Sankyo, Jazz Pharmaceuticals, Novartis, and Pfizer, outside the submitted work. P.M. reports grants, personal fees, and other from BMS, outside the submitted work. A.C.S. reports other from BMS/Celgene, during the conduct of the study; personal fees and other from AbbVie, Amgen, Astellas, BMS/Celgene, Novartis, and Pfizer; other from Glycomimetics and Servier; and personal fees from Jazz Pharmaceuticals and Teva, outside the submitted work. C.P. reports other from AbbVie, Amgen, Astellas, Janssen, Novartis, and Pfizer, outside the submitted work. A.H.W. reports grants and personal fees from AbbVie, Amgen, AstraZeneca, BMS/Celgene, Novartis, and Servier; grants from F. Hoffmann-La Roche; personal fees from Astellas, Genentech, Janssen, Macrogenics, and Pfizer; and other from Walter and Eliza Hall Institute, outside the submitted work. H.O. reports grants from Jazz Pharmaceuticals Denmark Aps; other from AbbVie A/S and Pfizer Aps, outside the submitted work. H.-J.K. reports personal fees and other from AbbVie, AIMS Bioscience, Amgen, AML-Hub, Astellas, BMS/Celgene, Daiichi Sankyo, Handok, Janssen, LG Chem, Novartis, Pfizer, Sanofi Genzyme, SL VaxiGen, and VigenCell; grants from BL&H; other from Boryung Pharma Co. and Pintherapeutics, outside the submitted work. R.A.L. reports personal fees and other from Celgene, during the conduct of the study. J.K. reports personal fees from AbbVie, Alexion, Amgen, Apellis, Celgene, Jazz Pharmaceuticals, and Novartis, outside the submitted work. F.T. reports other from AbbVie, Novartis, Pfizer, and Takeda; grants, research support, and other from BMS/Celgene; member of an advisory board for AbbVie, Astellas, BMS/Celgene, Novartis, Jazz Pharmaceuticals, and Pfizer, outside the submitted work. J.B. reports personal fees from Ariad/Incyte, BMS, Novartis, and Pfizer, outside the submitted work. O.S. reports personal fees from AbbVie, Celgene/BMS, Jazz Pharmaceuticals, Novartis, and Pfizer, outside the submitted work. H.D. reports grants and personal fees from AbbVie, Agios, Amgen, Astellas, BMS, Celgene, Jazz Pharmaceuticals, Novartis; personal fees from Astex Pharmaceuticals, AstraZeneca, Berlin-Chemie, GEMoaB, Gilead, Helsinn, Janssen, Oxford Biomedica, Roche, and Syndax; grants from Pfizer, outside the submitted work. A.T.F. reports grants and personal fees from AbbVie, Agios/Servier, and Celgene/BMS, during the conduct of the study; personal fees from Amgen, Amphivena, Astellas, Blueprint, Boston Biomedical, Daiichi Sankyo, Foghorn, Forma, Forty Seven, Genentech, Jazz Pharmaceuticals, Kite, Kura Oncology, Ipsen, Morphosys, NewLink Genetics, Novartis, Pfizer, PTC Therapeutics, Seattle Genetics, Takeda, Trillium, and Trovagene, outside the submitted work. E.L., X.Y., M.H., and P.M.-R. report employment and have equity interest in BMS. C.D.D. reports grants and personal fees from AbbVie, Agios, Celgene/BMS, Immune-Onc, Daiichi Sankyo, and Novartis; personal fees from Aprea, Foghorn, GSK, Notable Labs, and Takeda; grants from Calithera, outside the submitted work. The remaining authors declare no competing financial interests.
Figures

Comment in
-
IDH2 inhibition in AML.Blood. 2023 Jan 12;141(2):124-125. doi: 10.1182/blood.2022016946. Blood. 2023. PMID: 36633887 No abstract available.
References
-
- Cancer stat facts: leukemia - acute myeloid leukemia (AML). National Cancer Institute Surveillance Epidemiology and End Results Program. https://seer.cancer.gov/statfacts/html/amyl.html Available from:
-
- Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32(18):1919–1926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

