Hallmarks of DNA replication stress
- PMID: 35714587
- PMCID: PMC9219557
- DOI: 10.1016/j.molcel.2022.05.004
Hallmarks of DNA replication stress
Abstract
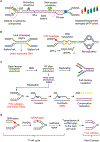
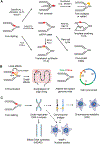
Faithful DNA replication is critical for the maintenance of genomic integrity. Although DNA replication machinery is highly accurate, the process of DNA replication is constantly challenged by DNA damage and other intrinsic and extrinsic stresses throughout the genome. A variety of cellular stresses interfering with DNA replication, which are collectively termed replication stress, pose a threat to genomic stability in both normal and cancer cells. To cope with replication stress and maintain genomic stability, cells have evolved a complex network of cellular responses to alleviate and tolerate replication problems. This review will focus on the major sources of replication stress, the impacts of replication stress in cells, and the assays to detect replication stress, offering an overview of the hallmarks of DNA replication stress.
Keywords: ATR; DNA damage; DNA repair; DNA replication; cancer; cell cycle; checkpoint; genomic integrity; genomic stability; replication stress.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests. L.Z. is a member of the advisory board of Molecular Cell.
Figures

References
-
- Aguilera A, and Garcia-Muse T (2012). R loops: from transcription byproducts to threats to genome stability. Mol Cell 46, 115–124. - PubMed
-
- Ait Saada A, Teixeira-Silva A, Iraqui I, Costes A, Hardy J, Paoletti G, Freon K, and Lambert SAE (2017). Unprotected Replication Forks Are Converted into Mitotic Sister Chromatid Bridges. Mol Cell 66, 398–410 e394. - PubMed
-
- Alabert C, Bukowski-Wills JC, Lee SB, Kustatscher G, Nakamura K, de Lima Alves F, Menard P, Mejlvang J, Rappsilber J, and Groth A (2014). Nascent chromatin capture proteomics determines chromatin dynamics during DNA replication and identifies unknown fork components. Nat Cell Biol 16, 281–293. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

