Astrocytes modulate neurodegenerative phenotypes associated with glaucoma in OPTN(E50K) human stem cell-derived retinal ganglion cells
- PMID: 35714595
- PMCID: PMC9287669
- DOI: 10.1016/j.stemcr.2022.05.006
Astrocytes modulate neurodegenerative phenotypes associated with glaucoma in OPTN(E50K) human stem cell-derived retinal ganglion cells
Abstract
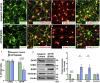
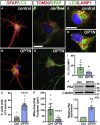
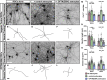
Although the degeneration of retinal ganglion cells (RGCs) is a primary characteristic of glaucoma, astrocytes also contribute to their neurodegeneration in disease states. Although studies often explore cell-autonomous aspects of RGC neurodegeneration, a more comprehensive model of glaucoma should take into consideration interactions between astrocytes and RGCs. To explore this concept, RGCs and astrocytes were differentiated from human pluripotent stem cells (hPSCs) with a glaucoma-associated OPTN(E50K) mutation along with corresponding isogenic controls. Initial results indicated significant changes in OPTN(E50K) astrocytes, including evidence of autophagy dysfunction. Subsequently, co-culture experiments demonstrated that OPTN(E50K) astrocytes led to neurodegenerative properties in otherwise healthy RGCs, while healthy astrocytes rescued some neurodegenerative features in OPTN(E50K) RGCs. These results are the first to identify disease phenotypes in OPTN(E50K) astrocytes, including how their modulation of RGCs is affected. Moreover, these results support the concept that astrocytes could offer a promising target for therapeutic intervention in glaucoma.
Keywords: astrocyte; autophagy; glaucoma; neurodegeneration; retina; retinal ganglion cell; stem cell.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Retinal Ganglion Cells With a Glaucoma OPTN(E50K) Mutation Exhibit Neurodegenerative Phenotypes when Derived from Three-Dimensional Retinal Organoids.Stem Cell Reports. 2020 Jul 14;15(1):52-66. doi: 10.1016/j.stemcr.2020.05.009. Epub 2020 Jun 11. Stem Cell Reports. 2020. PMID: 32531194 Free PMC article.
-
Acquisition of neurodegenerative features in isogenic OPTN(E50K) human stem cell-derived retinal ganglion cells associated with autophagy disruption and mTORC1 signaling reduction.Acta Neuropathol Commun. 2024 Oct 18;12(1):164. doi: 10.1186/s40478-024-01872-2. Acta Neuropathol Commun. 2024. PMID: 39425218 Free PMC article.
-
Exploring dysfunctional barrier phenotypes associated with glaucoma using a human pluripotent stem cell-based model of the neurovascular unit.Fluids Barriers CNS. 2024 Nov 14;21(1):90. doi: 10.1186/s12987-024-00593-x. Fluids Barriers CNS. 2024. PMID: 39543684 Free PMC article.
-
Altered Functions and Interactions of Glaucoma-Associated Mutants of Optineurin.Front Immunol. 2018 Jun 6;9:1287. doi: 10.3389/fimmu.2018.01287. eCollection 2018. Front Immunol. 2018. PMID: 29951055 Free PMC article. Review.
-
Retinal Ganglion Cells in a Dish: Current Strategies and Recommended Best Practices for Effective In Vitro Modeling of Development and Disease.Handb Exp Pharmacol. 2023;281:83-102. doi: 10.1007/164_2023_642. Handb Exp Pharmacol. 2023. PMID: 36907969 Free PMC article. Review.
Cited by
-
Evaluation of the LDN-0060609 PERK Inhibitor as a Selective Treatment for Primary Open-Angle Glaucoma: An In Vitro Study on Human Retinal Astrocytes.Int J Mol Sci. 2024 Jan 5;25(2):728. doi: 10.3390/ijms25020728. Int J Mol Sci. 2024. PMID: 38255802 Free PMC article.
-
Re-formation of synaptic connectivity in dissociated human stem cell-derived retinal organoid cultures.Proc Natl Acad Sci U S A. 2023 Jan 10;120(2):e2213418120. doi: 10.1073/pnas.2213418120. Epub 2023 Jan 4. Proc Natl Acad Sci U S A. 2023. PMID: 36598946 Free PMC article.
-
Advancing cell therapy for neurodegenerative diseases.Cell Stem Cell. 2023 May 4;30(5):512-529. doi: 10.1016/j.stem.2023.03.017. Epub 2023 Apr 20. Cell Stem Cell. 2023. PMID: 37084729 Free PMC article. Review.
-
Protection against Oxidative Stress by Coenzyme Q10 in a Porcine Retinal Degeneration Model.J Pers Med. 2024 Apr 22;14(4):437. doi: 10.3390/jpm14040437. J Pers Med. 2024. PMID: 38673065 Free PMC article.
-
RIP1 inhibition protects retinal ganglion cells in glaucoma models of ocular injury.Cell Death Differ. 2025 Feb;32(2):353-368. doi: 10.1038/s41418-024-01390-7. Cell Death Differ. 2025. PMID: 39448868 Free PMC article.
References
-
- Buckingham B.P., Inman D.M., Lambert W., Oglesby E., Calkins D.J., Steele M.R., Vetter M.L., Marsh-Armstrong N., Horner P.J. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J. Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

