CD49b identifies functionally and epigenetically distinct subsets of lineage-biased hematopoietic stem cells
- PMID: 35714596
- PMCID: PMC9287668
- DOI: 10.1016/j.stemcr.2022.05.014
CD49b identifies functionally and epigenetically distinct subsets of lineage-biased hematopoietic stem cells
Abstract
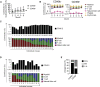
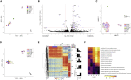
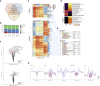
Hematopoiesis is maintained by functionally diverse lineage-biased hematopoietic stem cells (HSCs). The functional significance of HSC heterogeneity and the regulatory mechanisms underlying lineage bias are not well understood. However, absolute purification of HSC subtypes with a pre-determined behavior remains challenging, highlighting the importance of continued efforts toward prospective isolation of homogeneous HSC subsets. In this study, we demonstrate that CD49b subdivides the most primitive HSC compartment into functionally distinct subtypes: CD49b- HSCs are highly enriched for myeloid-biased and the most durable cells, while CD49b+ HSCs are enriched for multipotent cells with lymphoid bias and reduced self-renewal ability. We further demonstrate considerable transcriptional similarities between CD49b- and CD49b+ HSCs but distinct differences in chromatin accessibility. Our studies highlight the diversity of HSC functional behaviors and provide insights into the molecular regulation of HSC heterogeneity through transcriptional and epigenetic mechanisms.
Keywords: ATAC-seq; CD49b; HSC heterogeneity; RNA-seq; epigenetic regulation; hematopoietic stem cells; lineage bias; single-cell transplantation.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Adolfsson J., Borge O.J., Bryder D., Theilgaard-Mönch K., Åstrand-Grundström I., Sitnicka E., Sasaki Y., Jacobsen S.E.W. Upregulation of Flt3 expression within the bone marrow Lin−Sca1+c-kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

