New dawn for cancer cell death: Emerging role of lipid metabolism
- PMID: 35714911
- PMCID: PMC9237930
- DOI: 10.1016/j.molmet.2022.101529
New dawn for cancer cell death: Emerging role of lipid metabolism
Abstract
Background: Resistance to cell death, a protective mechanism for removing damaged cells, is a "Hallmark of Cancer" that is essential for cancer progression. Increasing attention to cancer lipid metabolism has revealed a number of pathways that induce cancer cell death.
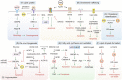
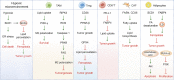
Scope of review: We summarize emerging concepts regarding lipid metabolic reprogramming in cancer that is mainly involved in lipid uptake and trafficking, de novo synthesis and esterification, fatty acid synthesis and oxidation, lipogenesis, and lipolysis. During carcinogenesis and progression, continuous metabolic adaptations are co-opted by cancer cells, to maximize their fitness to the ever-changing environmental. Lipid metabolism and the epigenetic modifying enzymes interact in a bidirectional manner which involves regulating cancer cell death. Moreover, lipids in the tumor microenvironment play unique roles beyond metabolic requirements that promote cancer progression. Finally, we posit potential therapeutic strategies targeting lipid metabolism to improve treatment efficacy and survival of cancer patient.
Major conclusions: The profound comprehension of past findings, current trends, and future research directions on resistance to cancer cell death will facilitate the development of novel therapeutic strategies targeting the lipid metabolism.
Keywords: Cancer; Cell death; Lipid metabolism; Therapeutic strategy.
Copyright © 2022 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures

Similar articles
-
Targeting dysregulated lipid metabolism in the tumor microenvironment.Arch Pharm Res. 2023 Dec;46(11-12):855-881. doi: 10.1007/s12272-023-01473-y. Epub 2023 Dec 7. Arch Pharm Res. 2023. PMID: 38060103 Free PMC article. Review.
-
Aberrant lipid metabolism as an emerging therapeutic strategy to target cancer stem cells.Stem Cells. 2020 Jan;38(1):6-14. doi: 10.1002/stem.3101. Epub 2019 Oct 31. Stem Cells. 2020. PMID: 31648395 Review.
-
Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention.Adv Drug Deliv Rev. 2020;159:245-293. doi: 10.1016/j.addr.2020.07.013. Epub 2020 Jul 23. Adv Drug Deliv Rev. 2020. PMID: 32711004 Free PMC article. Review.
-
Reprogramming of lipid metabolism in the tumor microenvironment: a strategy for tumor immunotherapy.Lipids Health Dis. 2024 Feb 1;23(1):35. doi: 10.1186/s12944-024-02024-0. Lipids Health Dis. 2024. PMID: 38302980 Free PMC article. Review.
-
Exosomes-regulated lipid metabolism in tumorigenesis and cancer progression.Cytokine Growth Factor Rev. 2023 Oct;73:27-39. doi: 10.1016/j.cytogfr.2023.05.002. Epub 2023 May 24. Cytokine Growth Factor Rev. 2023. PMID: 37291031 Review.
Cited by
-
The prognostic role and metabolic function of GGPS1 in oral squamous cell carcinoma.Front Mol Biosci. 2023 Mar 24;10:1109403. doi: 10.3389/fmolb.2023.1109403. eCollection 2023. Front Mol Biosci. 2023. PMID: 37033446 Free PMC article.
-
Fatty acid metabolism is related to the immune microenvironment changes of gastric cancer and RGS2 is a new tumor biomarker.Front Immunol. 2022 Dec 14;13:1065927. doi: 10.3389/fimmu.2022.1065927. eCollection 2022. Front Immunol. 2022. PMID: 36591293 Free PMC article.
-
Transcriptomics Reveals the Mechanism of Rosa roxburghii Tratt Ellagitannin in Improving Hepatic Lipid Metabolism Disorder in db/db Mice.Nutrients. 2023 Sep 28;15(19):4187. doi: 10.3390/nu15194187. Nutrients. 2023. PMID: 37836471 Free PMC article.
-
FABP5 suppresses colorectal cancer progression via mTOR-mediated autophagy by decreasing FASN expression.Int J Biol Sci. 2023 Jun 12;19(10):3115-3127. doi: 10.7150/ijbs.85285. eCollection 2023. Int J Biol Sci. 2023. PMID: 37416772 Free PMC article.
-
Knockdown of HOXD13 in Oral Squamous Cell Carcinoma Inhibited its Proliferation, Migration, and Influenced Fatty Acid Metabolism.J Cancer. 2025 Jan 1;16(1):214-226. doi: 10.7150/jca.102100. eCollection 2025. J Cancer. 2025. PMID: 39744569 Free PMC article.
References
-
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discovery. 2022;12(1):31–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

