Reaction Mechanism and Three-Dimensional Structure of GDP-d-glycero-α-d-manno-heptose 4,6-Dehydratase from Campylobacter jejuni
- PMID: 35715226
- PMCID: PMC9623766
- DOI: 10.1021/acs.biochem.2c00244
Reaction Mechanism and Three-Dimensional Structure of GDP-d-glycero-α-d-manno-heptose 4,6-Dehydratase from Campylobacter jejuni
Abstract
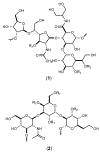
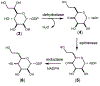
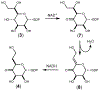
Campylobacter jejuni is a human pathogen and a leading cause of food poisoning in the United States and Europe. Surrounding the outside of the bacterium is a carbohydrate coat known as the capsular polysaccharide. Various strains of C. jejuni have different sequences of unusual sugars and an assortment of decorations. Many of the serotypes have heptoses with differing stereochemical arrangements at C2 through C6. One of the many common modifications is a 6-deoxy-heptose that is formed by dehydration of GDP-d-glycero-α-d-manno-heptose to GDP-6-deoxy-4-keto-d-lyxo-heptose via the action of the enzyme GDP-d-glycero-α-d-manno-heptose 4,6-dehydratase. Herein, we report the biochemical and structural characterization of this enzyme from C. jejuni 81-176 (serotype HS:23/36). The enzyme was purified to homogeneity, and its three-dimensional structure was determined to a resolution of 2.1 Å. Kinetic analyses suggest that the reaction mechanism proceeds through the formation of a 4-keto intermediate followed by the loss of water from C5/C6. Based on the three-dimensional structure, it is proposed that oxidation of C4 is assisted by proton transfer from the hydroxyl group to the phenolate of Tyr-159 and hydride transfer to the tightly bound NAD+ in the active site. Elimination of water at C5/C6 is most likely assisted by abstraction of the proton at C5 by Glu-136 and subsequent proton transfer to the hydroxyl at C6 via Ser-134 and Tyr-159. A bioinformatic analysis identified 19 additional 4,6-dehydratases from serotyped strains of C. jejuni that are 89-98% identical in the amino acid sequence, indicating that each of these strains should contain a 6-deoxy-heptose within their capsular polysaccharides.
Conflict of interest statement
The authors declare no competing conflicts of interests.
Figures





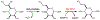
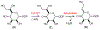
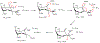
References
-
- Heimesaat MM, Backert S, Alter T, Bereswill S (2021) Human Campylobacteriosis-a serious infectious threat in a one health perspective. Curr Top Microbiol Immunol. 431, 1–23. - PubMed
-
- Dasti JI, Tareen AM, Lugert R, Zautner AZ, Gross U (2010) Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 300, 205–11. - PubMed
-
- Hermans D, Pasmans F, Messens W, Martel A, Immerseel FV, Rasschaert G, Heyndrickx M, Deun KV, Haesebrouck F (2012) Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 12, 89–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

