Oncology clinical trial disruption during the COVID-19 pandemic: a COVID-19 and cancer outcomes study
- PMID: 35715285
- PMCID: PMC9197329
- DOI: 10.1016/j.annonc.2022.04.071
Oncology clinical trial disruption during the COVID-19 pandemic: a COVID-19 and cancer outcomes study
Abstract
Background: COVID-19 disproportionately impacted patients with cancer as a result of direct infection, and delays in diagnosis and therapy. Oncological clinical trials are resource-intensive endeavors that could be particularly susceptible to disruption by the pandemic, but few studies have evaluated the impact of the pandemic on clinical trial conduct.
Patients and methods: This prospective, multicenter study assesses the impact of the pandemic on therapeutic clinical trials at two large academic centers in the Northeastern United States between December 2019 and June 2021. The primary objective was to assess the enrollment on, accrual to, and activation of oncology therapeutic clinical trials during the pandemic using an institution-wide cohort of (i) new patient accruals to oncological trials, (ii) a manually curated cohort of patients with cancer, and (ii) a dataset of new trial activations.
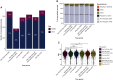
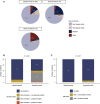
Results: The institution-wide cohort included 4756 new patients enrolled to clinical trials from December 2019 to June 2021. A major decrease in the numbers of new patient accruals (-46%) was seen early in the pandemic, followed by a progressive recovery and return to higher-than-normal levels (+2.6%). A similar pattern (from -23.6% to +30.4%) was observed among 467 newly activated trials from June 2019 to June 2021. A more pronounced decline in new accruals was seen among academically sponsored trials (versus industry sponsored trials) (P < 0.05). In the manually curated cohort, which included 2361 patients with cancer, non-white patients tended to be more likely taken off trial in the early pandemic period (adjusted odds ratio: 2.60; 95% confidence interval 1.00-6.63), and substantial pandemic-related deviations were recorded.
Conclusions: Substantial disruptions in clinical trial activities were observed early during the pandemic, with a gradual recovery during ensuing time periods, both from an enrollment and an activation standpoint. The observed decline was more prominent among academically sponsored trials, and racial disparities were seen among people taken off trial.
Keywords: COVID-19; cancer; clinical trials.
Copyright © 2022 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure The authors report the following conflicts of interest: ZB non-financial support, Bristol Myers Squibb (BMS); research support, Genentech/imCore. Honoraria from UpToDate. CL research support, Genentech/imCore. AS educational travel support, Pfizer and Astellas. MMA research support, BMS Lilly, AstraZeneca, and Genentech; consulting/advisory role, BMS, Lilly, AstraZeneca, Genentech, Merck, Achilles, and Abbvie. RH consulting/advisory role, BMS, Merck, Pfizer, Genetech, AstraZeneca, and GSK; research grant/funding, Merck, BMS, Pfizer, Genentech, GSK, and AstraZeneca. ST institutional research funding from AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Exelixis, BMS, Eisai, Nanostring, Cyclacel, Odonate, and Seattle Genetics; has served as an advisor/consultant to AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, BMS, Eisai, Nanostring, Puma, Sanofi, Celldex, Paxman, Puma, Silverback Therapeutics, G1 Therapeutics, AbbVie, Athenex, OncoPep, Outcomes4Me, Kyowa Kirin Pharmaceuticals, Daiichi-Sankyo, and Samsung Bioepsis Inc. MDG reports: Stock, Rappta Therapeutics; consulting/advisory role, BioMotiv, Janssen, Dendreon, Merck, GlaxoSmithKline, Lilly, Astellas, Genentech, BMS, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Alleron Therapeutics, Dracen, Inovio Pharmaceuticals, Numab, Dragonfly Therapeutics; institutional research funding, Janssen, Dendreon, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, Genentech/Roche. TKC research support, AstraZeneca, Alexion, Bayer, BMS/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, Takeda; honoraria, AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc. (Healthcare Communications Company with several brands such as OnClive, PeerView and PER), Research to Practice, L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, NEJM, Lancet Oncology, Heron Therapeutics, Lilly Oncology; consulting or advisory role, AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Heron Therapeutics, Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, Pionyr, Tempest, Lilly Ventures; Stocks: Pionyr, Osel, and Tempest; leadership role, Director of Genitourinary Oncology Division at Dana-Farber and past President of Medical Staff at Dana-Farber, member of NCCN Kidney panel and the GU Steering Committee, past chairman of the Kidney Cancer Association Medical and Scientific Steering Committee, KidneyCan Advisory board, Kidney cancer Research Summit co-chair (2019-); patents, royalties or other intellectual properties related to checkpoint inhibitors biomarkers and ctDNA (no royalties made as to date); travel, accommodations, expenses, in relation to consulting, advisory roles, or honoraria; medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies (ClinicalThinking, Envision Pharma Group, Fishawack Group of Companies, Health Interactions, Parexel, Oxford PharmaGenesis, and others). TKC’s institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter. TKC has mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components. DD Consulting/Advisory role, Mirati, Ipsen, Boehringer Ingelheim, Atheneum Partners, Boston Healthcare Associates, Dedham Group, Guidepoint Global Advisors; travel expenses, Ipsen. All other authors have declared no conflicts of interest.
Figures







References
-
- The New York Times Coronavirus World Map: Tracking the Global Outbreak – The New York Times. New York Times. 2020
-
- Schrag D., Hershman D.L., Basch E. Oncology practice during the COVID-19 pandemic. J Am Med Assoc. 2020;323(20):2005–2006. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

