Diethyl phthalate (DEP) perturbs nitrogen metabolism in Saccharomyces cerevisiae
- PMID: 35715465
- PMCID: PMC9205984
- DOI: 10.1038/s41598-022-14284-w
Diethyl phthalate (DEP) perturbs nitrogen metabolism in Saccharomyces cerevisiae
Abstract
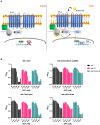
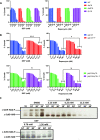
Phthalates are ubiquitously used as plasticizers in various consumer care products. Diethyl phthalate (DEP), one of the main phthalates, elicits developmental and reproductive toxicities but the underlying mechanisms are not fully understood. Chemogenomic profiling of DEP in S. cerevisiae revealed that two transcription factors Stp1 and Dal81 involved in the Ssy1-Ptr5-Ssy5 (SPS) amino acid-sensing pathway provide resistance to DEP. Growth inhibition of yeast cells by DEP was stronger in poor nitrogen medium in comparison to nitrogen-rich medium. Addition of amino acids to nitrogen-poor medium suppressed DEP toxicity. Catabolism of amino acids via the Ehrlich pathway is required for suppressing DEP toxicity. Targeted metabolite analyses showed that DEP treatment alters the amino acid profile of yeast cells. We propose that DEP inhibits the growth of yeast cells by affecting nitrogen metabolism and discuss the implications of our findings on DEP-mediated toxic effects in humans.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Staples CA, Peterson DR, Parkerton TF, Adams WJ. The environmental fate of phthalate esters: A literature review. Chemosphere. 1997;35(4):667–749. doi: 10.1016/S0045-6535(97)00195-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

