Leveraging a national biorepository in Zambia to assess measles and rubella immunity gaps across age and space
- PMID: 35715547
- PMCID: PMC9204687
- DOI: 10.1038/s41598-022-14493-3
Leveraging a national biorepository in Zambia to assess measles and rubella immunity gaps across age and space
Abstract
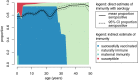
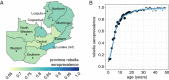
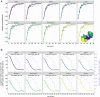
High-quality, representative serological surveys allow direct estimates of immunity profiles to inform vaccination strategies but can be costly and logistically challenging. Leveraging residual serum samples is one way to increase their feasibility. We subsampled 9854 residual sera from a 2016 national HIV survey in Zambia and tested these specimens for anti-measles and anti-rubella virus IgG antibodies using indirect enzyme immunoassays. We demonstrate innovative methods for sampling residual sera and analyzing seroprevalence data, as well as the value of seroprevalence estimates to understand and control measles and rubella. National measles and rubella seroprevalence for individuals younger than 50 years was 82.8% (95% CI 81.6, 83.9%) and 74.9% (95% CI 73.7, 76.0%), respectively. Despite a successful childhood vaccination program, measles immunity gaps persisted across age groups and districts, indicating the need for additional activities to complement routine immunization. Prior to vaccine introduction, we estimated a rubella burden of 96 congenital rubella syndrome cases per 100,000 live births. Residual samples from large-scale surveys can reduce the cost and challenges of conducting serosurveys, and multiple pathogens can be tested. Procedures to access quality specimens, ensure ethical approvals, and link sociodemographic data can improve the timeliness and value of results.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. At Least 80 Million Children Under One at Risk of Diseases Such as Diphtheria, Measles and Polio as COVID-19 Disrupts Routine Vaccination Efforts, Warn Gavi, WHO and UNICEF. https://www.who.int/news/item/22-05-2020-at-least-80-million-children-un... (2020).
-
- World Health Organization . Measles and Rubella Strategic Framework: 2021–2030. World Health Organization; 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

