Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the Phase III SPR1NT trial
- PMID: 35715566
- PMCID: PMC9205281
- DOI: 10.1038/s41591-022-01866-4
Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the Phase III SPR1NT trial
Abstract
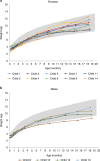
SPR1NT ( NCT03505099 ) was a Phase III, multicenter, single-arm study to investigate the efficacy and safety of onasemnogene abeparvovec for presymptomatic children with biallelic SMN1 mutations treated at ≤6 weeks of life. Here, we report final results for 14 children with two copies of SMN2, expected to develop spinal muscular atrophy (SMA) type 1. Efficacy was compared with a matched Pediatric Neuromuscular Clinical Research natural-history cohort (n = 23). All 14 enrolled infants sat independently for ≥30 seconds at any visit ≤18 months (Bayley-III item #26; P < 0.001; 11 within the normal developmental window). All survived without permanent ventilation at 14 months as per protocol; 13 maintained body weight (≥3rd WHO percentile) through 18 months. No child used nutritional or respiratory support. No serious adverse events were considered related to treatment by the investigator. Onasemnogene abeparvovec was effective and well-tolerated for children expected to develop SMA type 1, highlighting the urgency for universal newborn screening.
© 2022. The Author(s).
Conflict of interest statement
Novartis Gene Therapies, Inc., sponsored this clinical trial. The authors declare the following competing interests: K.A.S. has received personal compensation from Novartis Gene Therapies, Inc., (formerly AveXis) for serving as an advisory board member and from Biogen for serving as a visiting professor; and has received research support from Novartis Gene Therapies Inc., and Biogen-sponsored clinical trials. M.A.F. has received honoraria for scientific advisory boards from Novartis Gene Therapies, Inc., Biogen, and Roche, and research grants from Biogen. F. M. reports grants and personal fees from Novartis Gene Therapies, Inc., including participation in the STR1VE-EU grant and the SPR1NT study; grants, personal fees, and other (participation in the SHINE clinical trial and principal investigator of the investigator-initiated UK SMA REACH UK registry) from Biogen; and grants and personal fees from Roche, including participation in the JEWELFISH clinical trial of risdiplam and participation in the olesoxime clinical trial, during the conduct of the study. He has received honoraria for scientific advisory boards from Biogen, Novartis, Novartis Gene Therapies, Inc., PTC, Roche, and Sarepta. K. S. has served on advisory boards for Biogen, Novartis Gene Therapies, Inc., Novartis, and Chugai (Roche), and has received research funding from Biogen. J. R. M. has received personal compensation for clinical trial consulting and for serving on scientific advisory boards, as well as research support, from Novartis Gene Therapies, Inc. L.S. has received personal compensation as an advisory committee board member/consultant from Novartis Gene Therapies, Inc., Biogen, Biophytis, Cytokinetics, Dynacure, Roche, Santhera, and Sarepta Therapeutics, and has received research support from Novartis Gene Therapies, Inc., Biogen, Dynacure, and Roche. H. J. M. has received honoraria for scientific advisory boards from Novartis Gene Therapies, Inc., and research funding from Roche. R. S. F. has received personal compensation for consulting for advisory board participation from Novartis Gene Therapies, Inc., Biogen, Roche, and Scholar Rock, and for consulting from Novartis; editorial fees from Elsevier for co-editing a neurology textbook; license fees from the Children’s Hospital of Philadelphia; research funding from Novartis Gene Therapies, Inc., Biogen, Roche/Genentech, and Scholar Rock. K. J. S. has received personal compensation for a speaking engagement from Biogen, and research funding as a principal investigator for clinical trials sponsored by Novartis Gene Therapies, Inc., and Biogen. J. M. K. (Kwon) was her site’s principal investigator for clinical trials sponsored by Novartis Gene Therapies, Inc., Biogen, and Scholar Rock. C. M. Z. has received research support from Biogen. C. A. C. has served on advisory boards for Novartis Gene Therapies, Inc., Genentech, and Roche; served as an educational speaker for Biogen, and has received research funding from Novartis Gene Therapies, Inc., Biogen, and Roche. S. T. I. has received personal compensation for service on advisory boards or consulting from Novartis Gene Therapies, Inc., Biogen, Roche-Genetech, and Sarepta; and research support from Novartis Gene Therapies, Inc., Biogen, Capricor, PTC, Scholar Rock, and Sarepta. J. M. K. (Krueger) is a principal investigator for clinical trials sponsored by Novartis Gene Therapies, Inc., Scholar Rock, and Genentech-Roche. J. A. P. has served as an investigator on clinical trials for Biogen, Novartis, PTC Therapeutics, and Scholar Rock, and has served in advisory capacity for Biogen, Scholar Rock, Genentech Roche, and Novartis. P. B. S. has served as a consultant for Alexion, Biogen, Genentech, Novartis Gene Therapies, Inc., and Sarepta, and has served as a speaker for Alexion, Biogen, Genentech, Grifols, Novartis Gene Therapies, Inc., and PTC Therapeutics. S. K. is an employee of Novartis Gene Therapies, Inc., and receives consulting fees from UCB Pharma, Karuna Therapeutics, Worldwide Clinical Trials, CPC Clinical Research, Zosano Pharmaceuticals, PharPoint Research, and Nesos. S. T.-W. is an employee of Novartis Gene Therapies, Inc., and owns Novartis stock or other equities. B.E.M. is an employee of Translational Medicine, Novartis Institutes for BioMedical Research (Cambridge) and owns Novartis stock or other equities. T.A.M. is an employee of Novartis Gene Therapies, Inc., and owns Novartis stock or other equities.
Figures

Comment in
-
Early treatment is a lifeline for infants with SMA.Nat Med. 2022 Jul;28(7):1348-1349. doi: 10.1038/s41591-022-01889-x. Nat Med. 2022. PMID: 35840728 Free PMC article.
-
Timing is everything: Clinical evidence supports pre-symptomatic treatment for spinal muscular atrophy.Cell Rep Med. 2022 Aug 16;3(8):100725. doi: 10.1016/j.xcrm.2022.100725. Cell Rep Med. 2022. PMID: 35977471 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

