Measuring biological age using omics data
- PMID: 35715611
- PMCID: PMC10048602
- DOI: 10.1038/s41576-022-00511-7
Measuring biological age using omics data
Abstract
Age is the key risk factor for diseases and disabilities of the elderly. Efforts to tackle age-related diseases and increase healthspan have suggested targeting the ageing process itself to 'rejuvenate' physiological functioning. However, achieving this aim requires measures of biological age and rates of ageing at the molecular level. Spurred by recent advances in high-throughput omics technologies, a new generation of tools to measure biological ageing now enables the quantitative characterization of ageing at molecular resolution. Epigenomic, transcriptomic, proteomic and metabolomic data can be harnessed with machine learning to build 'ageing clocks' with demonstrated capacity to identify new biomarkers of biological ageing.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
T.W.-C. is a co-founder and scientific adviser of Alkahest and Qinotto. T.W.-C., J.R. and H.O. are co-founders and scientific advisers of Teal Omics.
Figures

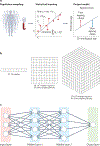

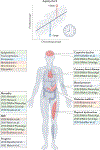
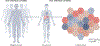
References
-
- Niccoli T & Partridge L Ageing as a risk factor for disease. Curr. Biol 22, R741–R752 (2012). - PubMed
-
-
López-Otín C, Blasco MA, Partridge L, Serrano M & Kroemer G The hallmarks of aging. Cell 153, 1194–1217 (2013).
This landmark review organized a framework to think about ageing through the lens of multiple conserved cellular and molecular processes and has become highly influential in the field of ageing research.
-
-
- Partridge L, Deelen J & Slagboom PE Facing up to the global challenges of ageing. Nature 561, 45–56 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

